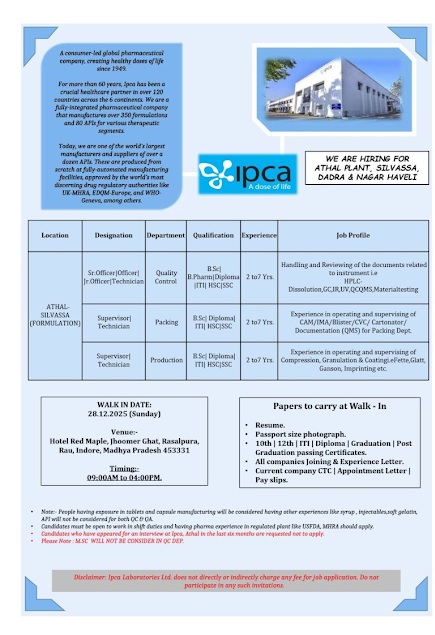
Ipca-Interview for BPharm, BSc in QC, Packing, Production on 28.12.2025
For more than 60 years, Ipca has been a crucial healthcare partner in over 120 countries across the 6 continents. We are a fully-integrated pharmaceutical company that manufactures over 350formulations and 80 APIs for various therapeutic segments. Today, we are one of the world’s largest manufacturers and suppliers of over a dozen APIs. These are produced from scratch at fully-automated manufacturing facilities, approved by the world’s most discerning drug regulatory authorities like UK-MHRA EDQM-Europe, and WHO- Geneva, among others.
Hiring for Athal Plant, Silvassa Dadra & Nagar Haveli
Sr.Officer / Officer / Jr.Officer / Technician
Department : Quality Control
Qualification : B.Sc / B.Pharm / Diploma / ITI / HSC / SSC
Experience : 2 to7 Yrs.
Job Profile : Handling and Reviewing of the documents related to instrument i.e HPLC- Dissolution,GC,IR,UV,QC QMS,Material testing
Supervisor / Technician
Department : Packing
Qualification : B.Sc / Diploma / ITI / HSC / SSC
Experience : 2 to7 Yrs.
Job Profile : Experience in operating and supervising of CAM/IMA/Blister/CVC/ Cartonator/ Documentation (QMS) for Packing Dept.
Supervisor / Technician
Department : Production
Qualification : B.Sc / Diploma / ITI / HSC / SSC
Experience : 2 to 7 Yrs.
Job Profile : Experience in operating and supervising of Compression, Granulation & Coatingi.eFette,Glatt, Ganson, Imprinting etc.
WALK IN INTERVIEW
Date : 28.12.2025 (Sunday)
Venue : Hotel Red Maple, Jhoomer Ghat, Rasalpura, Rau, Indore, Madhya Pradesh 453331
Timings : 09:00AM to 04:00PM.
Papers to carry at Walk – In
• Resume.
• Passport size photograph.
• 10th | 12th | ITI | Diploma | Graduation | Post
• Graduation passing Certificates.
• All companies Joining & Experience Letter.
• Current company CTC | Appointment Letter |
• Pay slips.
People having exposure in tablets and capsule manufacturing will be considered having other experiences like syrup, injectables,soft gelatin, API will not be considered for both QC & QA.
• Candidates must be open to work in shift duties and having pharma experience in regulated plant like USFDA, MHRA should apply.
• Candidates who have appeared for an interview at Ipca, Athal in the last six months are requested not to apply.
• Please Note : M.SC WILL NOT BE CONSIDER IN QC DEP.