An Introduction to Clinical Trials
What is Clinical Trials?
A properly planned and executed clinical trial is a powerful experimental technique for assessing the effectiveness of an intervention
What is an investigational product?
‘a pharmaceutical form of an active substance or placebo being tested or used as a reference in a clinical trial, including products already with a marketing authorisation but used or assembled (formulated or packaged) in a way different from the authorised form, or when used for an unauthorised indication, or when used to gain further information about the authorised form’
What makes Clinical Trial different from ‘Standard of Care’
- Involves human subjects
- Test an ‘intervention’ – be it a product, procedure or health care sytem….in order to improve standard of care!
- Measures effects over a period of time
- Most have a comparison CONTROL group
- Must have method to measure intervention
– this is captured in the protocol and this must be stuck to meticulously if the question is to be answered!!
- Focuses on unknowns: effect of intervention
- Must be done before medication is part of standard of care
- Standard of Care all about clinical judgement decision/flexibility – trials need all to stick with the protocol, no deviation – within your clinical judgement
Why Do Research Studies?
To collect data on usual and unusual events, conditions, & population groups
To test hypotheses formulated from observations and/or intuition
Ultimately, to understand better – improve health outcomes with change
Types of Medical Research Studies
Non-directed Data Capture
- Vital Statistics
Directed Data Capture & Hypothesis Testing
- Cohort Studies, Case Control Studies
Clinical Trials
- Investigation of Treatment/Condition
- Drug Trials
What makes Clinical Trial different from ‘Standard of Care’
- Involves human subjects
- Test an ‘intervention’ – be it a product, procedure or health care sytem….in order to improve standard of care!
- Measures effects over a period of time
- Most have a comparison CONTROL group
- Must have method to measure intervention
– this is captured in the protocol and this must be stuck to meticulously if the question is to be answered!!
- Focuses on unknowns: effect of intervention
- Must be done before medication is part of standard of care
- Standard of Care all about clinicial judgment decision/flexibility – trials need all to stick with the protocol, no deviation – within your clinical judgment
Some Examples of Trials….
They could be small investigator-led fellowship type studies that are addressing a disease management question, through to large multi-centre programmes within collaborations or with product development sponsors assessing new products for licensure
They might be ward based….
Improving disease management in very sick children such as severe malaria, malnutrition and management of seizures and in-patient trials for product development such as PK studies
Or Community Based…
Phase II and III regulatory trials in drug and vaccines for malaria and HIV. Academic proof of concept trails. Large phase IV surveillance studies.
So are trials a good thing, have they improved healthcare?
Formal record of clinical trials dates back to the time of the “Trialists”:
- Dr. Van Helmont’s proposal for a therapeutic trial of bloodletting for fevers [1628]
- Dr. Lind’s, a ship surgeon, trial of oranges & limes for scurvy [1747]
Historical Highlights of Drug Trials
- 1909: Paul Ehrlich – Arsphenamine
- 1929: Alexander Fleming – Penicillin
- 1935: Gerhard Domagk – Sulfonamide
- 1944: Schatz/Bugie/Waksman – Streptomycin
- By 1950, the British Medical Res. Council developed a systematic methodology for studying & evaluating therapeutic interventions
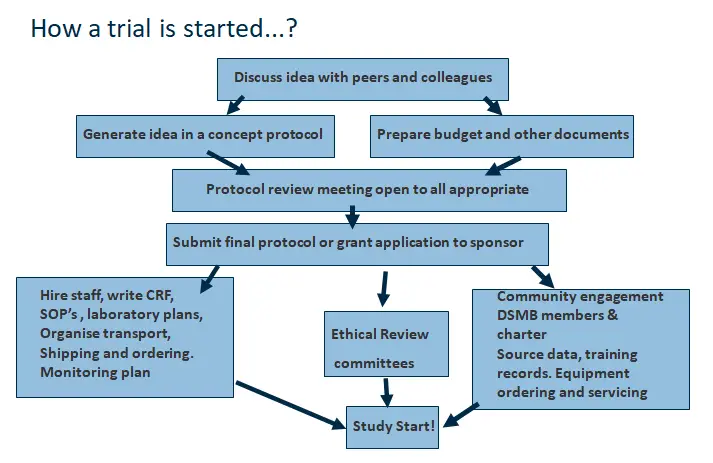
So where do I start?
Basic Concepts
- The protocol. Establishes the question – ideally has just one and this is the primary end point. Common failing is too many end points. The best designed trials keep it simple as this make a clear answer more likely and easier to acheive
- Secondary objectives; a few related, appropriate secondary questions are normal as long as they do not distract from the primary. Some might be exploratory.
- Trial is then designed around these. The protocol sets out how the question will be answered
The protocol….all in the title
- Single centre, placebo controlled etc etc
- Who is conducting the trial, who is sponsoring it, where is it to be conducted and on whom will you be conducting the research
- What are you testing? Is it safe, have the tests been validated? Why is this research needed.
- What are the risks, what are the procedures, how will data be collected. How did you calculate how many patients you will need.
Informed Consent Form
- As it says … a form by which you gain ‘informed consent’
- Few key requirements which must be included. Very difficult balance … examples of 17 page forms. Still ‘informed’ consent?
- In Swahili ‘research’ also means ‘explorative test’ therefore difficult to explain difference between standard of care and research – this is a key principle of giving consent.
- Special circumstances – children and emergency. What about this setting? Really so different? When do you need a witness?
- Whole point of GCP is to protect the rights of the subject
The Case Record Form
- Turns the protocol into your data capture system
- Should only collect data listed in the protocol and nothing else… i.e unless you will use ‘weight’ and have set out to do so, no need to record. Often far too long and collects data that is not used.
- Differs from the source data – patient notes and lab reports. This is a central concept in GCP that data is always verifiable
- Data taken from here and entered into a database and then exported to statistical package. Important to keep CRFs to allow you to go back and resolve data queries
Database and Statistics
- Likely to need stats advice right at the start to help you decide on the all important ‘n’…. How will you randomise, maybe you don’t need 1:1. Keeping the numbers down is helpful. Time, cost and ethics – but you still need to answer the question
- Protocol needs to explain statistical objectives of your trial but it is the report and analysis plan that sets out how you will analysis the data. Must be finalised before database close to avoid risk of manipulating the data
- Database should be secure and have an audit trial. Currently difficult in non-commercial trials
Keys to following the Protocol….The Case Record Form, Source Data and SOP’s.
- The Case Record form turns the protocol into a data capture system
- Should only collect data listed in the protocol and nothing else… i.e unless you will use ‘weight’ and have set out to do so, no need to record. Often far too long and collects data that is not used.
- Differs from the source data – patient notes and lab reports. This is a central concept in GCP that data is always verifiable
- Data taken from here and entered into a database and then exported to statistical package. Important to keep CRFs and source data to allow you to go back and resolve data queries
- Operations manuals or ‘SOP’s translate the protocol to the practical and operational steps appropriate to your site
Who is involved?
- Investigators
- Coordinators / Project managers
- Nurses, clinical officers, fieldworkers
- Pharmacists
- Data manager and entry clerks
- Monitor / QA
- Laboratory staff
And possibly….
Why did we need recognised international guidelines for conducting trials?
- Following famous cases such as the Nazis in WWII and black American men in syphilis studies (1932 –1972) there followed the declaration of Helsinki
- Agreement between countries that there needed to be a global standard by which all trial are conducted
- This is Good Clinical Practice – protects those in a trial, but also those who’s treatment will depend on the data
- Essentially ensures that the rights of the patient are protected and by all those given a drug or intervention in the future based upon that data
Definition of ICH-GCP
“ a standard for the design, conduct, performance, monitoring, auditing, recording, analyses, and reporting of clinical trials that provides assurance that the data and reported results are credible and accurate, and that the rights, integrity and confidentiality of trial subjects are protected.”
(ICH GCP)
Definition
- Quality Data + Ethics = GCP
- Data and Reported Results are Credible, and Accurate = quality data
- Rights, Integrity, and Confidentiality of Trial Subjects are Protected = ethics
The basics on how to comply with GCP
- 1. Write a good protocol -Weigh risks vs. benefits
- Obtain IRB/IEC approvals
- Protect the subjects –
–Obtain Informed Consent,
–Ensure safety, rights & confidentiality
- Use qualified study team
- Handle investigational products appropriately
- Implement quality systems
- Record and analyze information appropriately
- Follow the protocol and trial SOP’s!!!!
Other things to think about
- Clinical trial insurance / Non-negligent harm cover
- Safety reporting
- Ethics committee safety and annual updates
- Clinical trial registries
- Sponsor reports
- Publication planning
- Logistics, transport, budgeting
- Drug/vaccine storage
- Sample transportation, export, storage
- Data archiving
- SOP’s, training records and equipment service contracts
Too daunting, are you put off completely?
- Don’t be!
- Excellent way to learn about research – plenty of help… and plenty of funding out there
- More money than ever going into capacity building for clinical trials in resource limited settings
- Many opportunities for training and further qualifications
- Great field of research – whatever your training. Getting an answer to a trial could influence the way patients are managed or make a new drug/vaccine available. The possibility for improving health outcomes for thousands rather than one patient in front of you