Types of liquid column chromatography( HPLC)
The type of liquid column chromatography (HPLC) can be classified on the nature of the stationary phase and the separation process. There Can be classified into three types
- Adsorption chromatography
- Ion-exchange chromatography
- Size exclusion chromatography
Adsorption chromatography
In adsorption chromatography the stationary phase is an adsorbent (like silica gel or any other silica based packing’s) and the separation is based on repeated adsorption-desorption steps.
Ion-exchange chromatography
In ion-exchange chromatography the stationary bed has an ionically charged surface of opposite charge to the sample ions. This technique is used almost exclusively with ionic or ionizable samples. The stronger the charge on the sample, the stronger it will be attracted to the ionic surface and thus, the longer it will take to elute. The mobile phase is an aqueous buffer, where both pH and ionic strength are used to control elution time.
Size exclusion chromatography
In size exclusion chromatography the column is filled with material having precisely controlled pore sizes, and the sample is simply screened or filtered according to its solvated molecular size. Larger molecules are rapidly washed through the column; smaller molecules penetrate inside the porous of the packing particles and elute later. Mainly for historical reasons, this technique is also called gel filtration or gel permeation chromatography although, today, the stationary phase is not restricted to a “gel”.
Concerning the first type, two modes are defined depending on the relative polarity of the two phases: normal and reversed-phase chromatography.
- Normal phase chromatography
- Reversed-phase chromatography
Normal phase chromatography
In normal phase chromatography, the stationary bed is strongly polar in nature (e.g., silica gel), and the mobile phase is nonpolar (such as n-hexane or tetrahydrofuran). Polar samples are thus retained on the polar surface of the column packing longer than less polar materials.
Reversed-phase chromatography
Reversed-phase chromatography is the inverse of this. The stationary bed is nonpolar (hydrophobic) in nature, while the mobile phase is a polar liquid, such as mixtures of water and methanol or acetonitrile. Here the more nonpolar the material is, the longer it will be retained.
Above mentioned all types of HPLC cover almost 90% of all chromatographic applications. Eluent polarity plays the highest role in all types of HPLC. There are two elution types: isocratic and gradient. In the first type constant eluent composition is pumped through the column during the whole analysis. In the second type, eluent composition (and strength) is steadily changed during the run.
Overlay of the four components trace analysis chromatograms. (A) is the isocratic elution, (B) is the gradient elution, shadow line is the gradient profile from 30% acetonitrile in water to 65% acetonitrile.
HPLC as compared with the classical technique is characterized by:
small diameter (2-5 mm), reusable stainless steel columns
column packings with very small (3, 5 and 10 mm) particles and the continual development of new substances to be used as stationary phases
relatively high inlet pressures and controlled flow of the mobile phase
precise sample introduction without the need for large samples
special continuous flow detectors capable of handling small flow rates and detecting very small amounts
automated standardized instruments
automated standardized instruments
rapid analysis; and high resolution
Note:
Initially, pressure was selected as the principal criterion of modern liquid chromatography and thus the name was “high pressure liquid chromatography” or HPLC. This was, however, an unfortunate term because it seems to indicate that the improved performance is primarily due to the high pressure.
This is, however, not true. In fact high performance is the result of many factors:
- Very small particles of narrow distribution range and
- Uniform pore size and distribution,
- High pressure column slurry packing techniques,
- Accurate low volume sample injectors,
- Sensitive low volume detectors and
- Good pumping systems.
Naturally, pressure is needed to permit a given flow rate of the mobile phase; otherwise, pressure is a negative factor not contributing to the improvement in separation. Recognizing this, most experienced chromatographers today, refer to the technique as high performance liquid chromatography still permitting the use of the acronym HPLC.
Liquid chromatography Column Separation
The separation occurs inside the LC column. You may be wondering what is happening inside the column. Let’s use “after-work activity” as an example to explain the column separation.
The LC column is filled with very small particles (also called gels), and the size of gel is 3 to 15μm (μm=1/1000mm) or smaller. This gel has various “traps”. Each sample component has different “characteristics” and interacts with the “trap” differently, i.e., each component may stay inside the column for different lengths. Thus using this time differences, the components are separated.
The condition change, in case of LC, is to change compositions of mobile phase, pH, column temperatures etc. By changing the conditions, the components which were not able to be separated may be separated or could be vice versa. This is the difficult point of LC and where the user needs to pay a big attention. In some cases the same “trap” with different conditions may make the separation possible, but in other cases, the same “trap” does not work at any conditions you try.
In the latter case, different kind of trap may be required. In “LC word”, the former means that using the same column with different conditions (mobile phase/ temperature etc.) and the latter case means to change the LC column. There are different LC column packing materials (base material) available. Moreover using different modifications (addition of chemical compounds on the surface of the gel), a wide variety of LC column can be prepared.
HPLC Separation
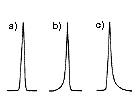
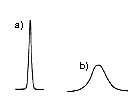
LC separation result should obtained a symmetrical peak shape (Figure 1a). When there is a problem, the peak will not be a symmetrical one and may show leading (Figure 1b) or tailing (Figure 1c). The high-performance column will provide narrower peak (Figure 2a) and low-performance column will provide wider peak (Figure 2b).
To measure the performance of the column, we use “theoretical plate number (TPN)”. It can be said that the bigger the TPN, the better the column. TPN is directly proportional to the column length, i.e., if the column length was doubled or two columns were used in a series, the TPN is also doubled. The internal diameter of the column also influences the TPN, but it is not as significant as that of column length. Also TPN may differ if different LC settings or measurement methods were used, even using the same column and the same mobile phase.
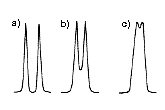
For the separation of two components in the sample, it is ideal to have separation at the baseline level (Figure 3a). When two peaks are too close, they may overwrap, and results in insufficient separation (Figure 3b-c). The column with higher TPN provides sharper peaks, thus the possibility of overwrapping is smaller than the column with lower TPN.
 |
 |
 |
| (a) Normal peak (b) Leading (c) Tailing |
(a) High TPN (b) Low TPN |
(a) Baseline separation (b) Some peak overwrapping (c) Poor separation |
| Figure 1. Examples of peak shapes. | Figure 2. Peak shapes and column TPN. | Figure 3. Separation of two components. |
Even when peak overwrapping was observed, this may be solved by changing the analytical conditions (e.g. changing mobile phase). If the separation cannot be improved by the conditional changes, different types of LC column may be used.
For the qualification analysis (to identify what components are present in the sample), peak overwrapping may not be a big concern. However, for the quantification analysis (to measure how much each sample is present in the sample), the baseline separation is required for the precise measurement.
Types of packed gels
Silica gel
Silica gel is Silica, silicon dioxide, has the chemical formula of SiO2. A small paper bag of silica in food packages stated ‘do not eat’. It is used as a dehydrator. The ones used for dehydrator has a gel diameter of 1 mm or larger, but the ones packed in LC columns are very small; few um sizes.
There are two types of silica gels.
- Spherical shapes
- Irregular shapes
the spherical shaped gels are most widely used these days. The silica gel used in LC has pores on the surface of the gel. By having the pores, it provides larger surface area compared to the ones without pores. The size of pore is very small and expressed in angstrom (Å) unit. The silica with pores is called porous silica.
There are few indexes used to express silica gel grades.
Shape : Most silica columns used nowadays contain spherical type.
Size : Smaller size particles have been developed. Current major line is 5μm, but even smaller size 1.5 to 3μm gel is also in use. The smaller gels are packed in smaller column housing and thus decreases the analytical time.
Pore size : There is not a simple good/bad indicator for pore sizes. The right pore size should be determined depending on the size of target analyte.
Surface area : This is the relative surface area of the gel. The smaller the particle size, the relative surface area becomes larger. Also the larger the number of pores, the larger the relative surface area. If all the other indexes are the same, the better performance can be expected from the larger surfaced-area gel. One gram of conventional silica gel provides a surface area of softball field.
Polymer gel
Polymer-based column is becoming more popular nowadays. The known polymers include polyethylene and poly propylene. Columns that is use different types of polymers some are as below.
(1) Polystylene (Styrene divinylbenzene copolymer)
(2) Polymethacrylate
(3) Polyhydroxymethacrylate
(4) Polyvinyl alcohol
Similar to the silica gel, the polymer gel is manufactured into very small particles.
Other gel
Other than silica and polymer gels, the gels used include natural substances such as cellulose, agarose, dextrin, and chitosan, and members of ceramics such as hydroxyapatite and zirconia. However, their use is very limited.
Types of separation mode
Many different types of “traps” in the column, and depending on the “trap” there are different types of columns. This “trap” is called separation mode. Generally used separation modes in LC are listed below.
- Reversed-phase (RP) mode
- Normal-phase (NP) mode
- Hydrophilic Interaction (HILIC) mode
- Ion exchange (IE) mode
- Ligand exchange mode
- Ion exclusion mode
- GPC mode
- GFC mode
- Multi mode
- Affinity mode
- Chiral mode
HPLC Chromatography troubleshooting
For more pharma update Please click here-www.pharmaguidances.com

