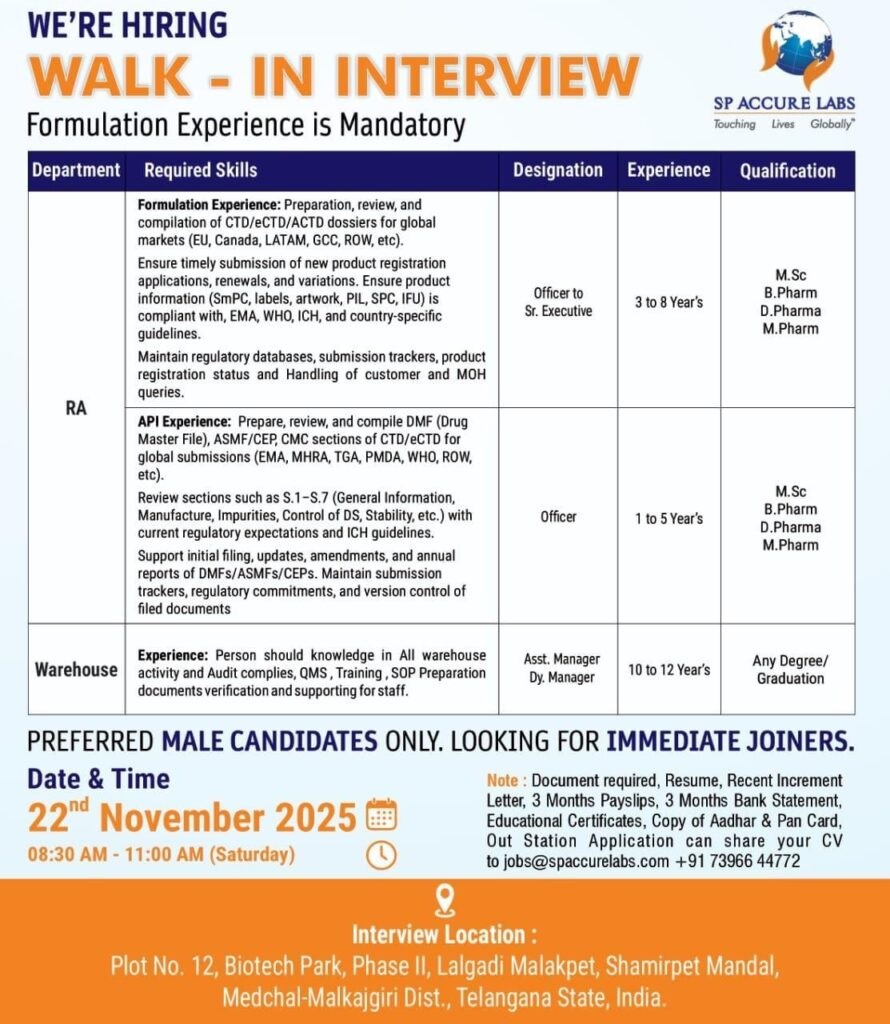
SP Accure Labs- Walk-In Interview for RA & Warehouse Roles on 22nd November 2025
Regulatory Affairs (RA) – Formulation Experience Required
a) Officer to Sr. Executive (3 to 8 Years)
Required Skills:
Preparation, review, and compilation of CTD/eCTD/ACTD dossiers for global markets including EU, Canada, LATAM, GCC, ROW
Submission of new product registration applications, renewals, and variations
Ensuring product information (SmPC, PIL, labels, artwork, SPC, IPC, IFU) meets EMA, WHO, ICH, and country-specific guidelines
Maintaining regulatory databases, submission trackers, and handling customer & MOH queries
Qualification:M.Sc / B.Pharm / D.Pharm / M.Pharm
b) Officer – API RA (1 to 5 Years)
Required Skills:
Preparation and review of DMFs, ASMFs, CEPs, and CMC sections for CTD/eCTD submissions (EMA, MHRA, TGA, PMDA, WHO, ROW)
Knowledge of S.1–S.7 modules: General Information, Manufacturing, Impurities, Control of DS, Stability
Support for filing, amendments, and annual reports
Maintaining submission trackers, version control, and regulatory commitments
Qualification:M.Sc / B.Pharm / D.Pharm / M.Pharm
2. Warehouse Department
Asst. Manager / Dy. Manager (10 to 12 Years)
Required Skills:
•Strong knowledge of warehouse operations
•Experience in QMS, training, SOP preparation, audit activities, and documentation
•Supporting staff and managing document verification processes
Qualification:Any Degree / Graduation
📅 Date: 22nd November 2025 (Saturday)
⏰ Time: 08:30 AM to 11:00 AM
📍 Interview Location:
Plot No. 12, Biotech Park, Phase II, Lalgadi Malakpet,
Shamirpet Mandal, Medchal-Malkajgiri District, Telangana, India.
Important Notes
Preferred Candidates: Male candidates only