Tablets Lubricants Lubricants and Glidants used in Tablet Formulations are: • Magnesium stearate • Stearic acid • Sodium stearyl fumarate • Hydrogenated vegetable oil • PEG 4000, 6000 • Hexagonal boron nitride • DL-Leucine • Sodium lauryl sulfate • Gliceryl behenate • Sodium benzoate • Colloidal silicone dioxide • Talc …
Read More »Pharmaguidances Whats App Groups
Pharmaguidances Whats App Groups Pharmaguidances Pharma Post and Jabs Pharma Vacancy Pharma Post and Jabs 2 Pharmaguideline Pharmaguideline 2 MFR of SALBUTAMOL SULPHATE TABLET
Read More »Preformulation stability studies
Stability Analysis Preformulation stability studies are the first quantitative assessment of chemical stability of a new drug. This may involve 1. Stability study in toxicology formulation 2. Stability study in solution state 3. Stability study in solid state. Stability Study in Toxicology Formulation: A new drug is administered to animals …
Read More »Powder Particle Size Determination Methods for tablets
Particle Size Determination Methods: 1. Sieving 2. Microscopy 3. Sedimentation rate method 4. Light energy diffraction 5. Laser holography 6. Cascade impaction 1. Sieving method: • Range: 50 – 150 μm • Simple, inexpensive • If powder is not dry, the apertures get clogged. 2. Microscopy: • Range: 0.2 – …
Read More »Powder flow properties in Pharmaceuticals
Powder flow properties in Pharmaceuticals Apparent bulk density (g/cm3): Bulk drug powder is sieved through 40 mesh screen. Weight is taken and poured into a graduated cylinder via a large funnel. The volume is called bulk volume. Apparent bulk density = Weight of the powder/Bulk volume Tapped density (g/cm3): Bulk …
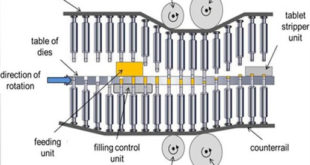
Read More »TABLET TOOLING
TABLET TOOLING Basics of Tablet Tooling Tablet compression machines are made keeping in view the type of dies and punches will be used on them. The dies and punches and their setup on compression machine is called tooling, it is classified as B and D mainly. The B tooling dies …
Read More »Preformulation for Tablets, Capsules, Liquid Orals
Preformulation for Tablets, Capsules, Liquid Orals Before developing a formulation like tablets, capsules, liquid orals we study the suitability of new drug or drug and excipients for the chosen formulation which is called preformulation. Preformulation Definition Preformulation may be defined as a stage of the research and development process where …
Read More »Pharmaceutical Quality Assurance -Drug and Therapeutics Committee Training Course
Pharmaceutical Quality Assurance -Drug and Therapeutics Committee Training Course CONTENTS Pharmaceutical Quality Assurance Acknowledgment Purpose and Content Objectives Outline Key Definitions Introduction Determinants or Aspects of Medicine Quality Critical Elements of a Comprehensive Quality Assurance Program How Is Quality Assessed? How Is Quality Assured? Who Ensures Medicine Quality? Pharmaceutical Quality …
Read More »MONOGRAPH ON LIQUID PREPARATIONS FOR ORAL USE AS PER INTERNATIONAL PHARMACOPOEIA
MONOGRAPH ON LIQUID PREPARATIONS FOR ORAL USE AS PER INTERNATIONAL PHARMACOPOEIA Definition Liquid preparations for oral use are usually solutions, emulsions or suspensions containing one or more active ingredients in a suitable vehicle, they may in some cases consist simply of a liquid active ingredient used as such. Liquid preparations …
Read More »SOP on Testing Efficacy of Disinfectants
SOP on Testing Efficacy of Disinfectants. Objective: To lay down a procedure for testing efficacy of Disinfectants. Scope : This SOP is applicable for testing of efficacy of disinfectant solutions used in microbiology laboratory and production area. Responsibility Microbiologist Head-Microbiology Accountability Head-Quality Control Head-Quality Assurance Procedure Prepare Culture suspension of …
Read More »Lorazepam Aristo 2.5 mg tablets
Lorazepam Aristo 2.5 mg tablets Active ingredient – lorazepam Legal Category : POM: Prescription only medicine 1. Name of the medicinal product Lorazepam Aristo 2.5 mg tablets 2. Qualitative and quantitative composition One tablet contains 2.5 mg lorazepam. Excipients with known effect Each tablet contains 116.7 mg lactose (as lactose …
Read More »Aciclovir 400 mg Tablets
Aciclovir 400 mg Tablets Active ingredient – Aciclovir Legal Category -POM: Prescription only medicine 1. Name of the medicinal product Aciclovir 400 mg Tablets 2. Qualitative and quantitative composition Each 400 mg tablet contains 400 mg aciclovir. For the full list of excipients, see section 6.1 3. Pharmaceutical form Tablet …
Read More »Furosemide 40 mg Tablets
Furosemide 40 mg Tablets Active ingredient – Furosemide Legal Category – POM: Prescription only medicine 1. Name of the medicinal product Furosemide 40mg Tablets 2. Qualitative and quantitative composition Each tablet contains Furosemide 40mg. For a full list of excipients, see section 6.1. 3. Pharmaceutical form Tablet White to off white, …
Read More »SCHEDULE G – (THE DRUGS AND COSMETICS ACT 1940 AND RULES 1945 )
SCHEDULE G – (THE DRUGS AND COSMETICS ACT 1940 AND RULES 1945 ) Aminopterin L-Asparaginase Bleomycin Busulphan; its salts Carbutamide Chlorambucil;its salts Chlorothiazide and other derivatives of 1, 2, 4 benzothiadiazine Chlorpropamide; its salts Chlorthalidone and other derivatives of Chlorobenzene compound. Cis-Platin Cyclophosphamide; its salts Cytarabine Daunorubicin Di-Isopropyl Eluorophosphate Disodium …
Read More »Labelling of medicines (Drugs and Cosmetics Act ,1940 and Rules, 1945)
Labelling of medicines (Drugs and Cosmetics Act ,1940 and Rules, 1945) (1) The container of a medicine for internal use shall— (a) if it contains a substance specified in Schedule G, be labelled with the words ―Caution: it is dangerous to take this preparation except under medical supervision‖ – conspicuously …
Read More »Packing of drugs (Drug & Cosmatic act 1940 Rules 1945)
Packing of drugs (Drugs and Cosmetics Act ,1940 and Rules, 1945) (1) The pack sizes of drugs meant for retail sale shall be as prescribed in Schedule P1 to these rules. (2) The pack sizes of drugs not covered by Schedule P-1 shall be as given below: – Unless specified …
Read More »SCHEDULE H1 ( DRUGS AND COSMETICS ACT 1940 AND RULES 1945)
SCHEDULE H1 ( DRUGS AND COSMETICS ACT 1940 AND RULES 1945) 1.Alprazolam 2.Balofloxacin 3.Buprenorphine 4.Capreomycin 5.Cefdinir 6.Cefditoren 7.Cefepime 8.Cefetamet 9.Cefixime 10.Cefoperazone 11.Cefotaxime 12.Cefpirome 13Cefpodoxime 14.Ceftazidime 15.Ceftibuten 16.Ceftizoxime 17.Ceftriaxone 18.Chlorodiazepoxide 19.Clofazimine 20.Codeine 21.Cycloserine 22.Diazepam 23.Diphenoxylate 24.Doripenam 25.Ertapenem 26.Ethambutol Hydrochloride 27.Ethionamide 28.Feropenam 29.Gemifloxacin 30.Imipenem 31.Isoniazide 32.Levofloxacin 33.Meropenem 34.Midazolam 36.Moxifloxacin 37.Nitrazepam 38.Pentazocine 39.Prulifloxacin …
Read More »SCHEDULE – H (PRESCRIPTION DRUGS) – (THE DRUGS AND COSMETICS ACT 1940 AND RULES 1945 )
SCHEDULE – H (PRESCRIPTION DRUGS) – (THE DRUGS AND COSMETICS ACT 1940 AND RULES 1945 ) Abacavir Abciximab Acamprosate calcium Acebutol hydrochloride Aclarubicin Albendazole Alclometasone dipropionate Actilyse Acyclovir Adenosine Adrenocorticotrophic hormone (acth) Alendronate sodium Alopurinol Alphachymotrypsin Alprazolam Alprostadil Amantadine hydrochloride Amifostine Amikacin sulphate Amiloride hydrochloride Amineptine Aminoglutethimide Amino salicylic acid …
Read More »Manner of Labelling (THE DRUGS AND COSMETICS ACT 1940 AND RULES 1945 )
Manner of Labelling .— (1) Subject to the other provisions of these Rules, the following particulars shall be either printed or written in indelible ink and shall appear in a conspicuous manner on the label of the innermost container of any drug and on every other covering in which the …
Read More »SOP on Personnel Qualification for Aseptic Processing and Sterility Testing Area
Personnel Qualification for Aseptic Processing and Sterility Testing Area Objective: To lay down the procedure for Personnel Qualification to enter and work in the Aseptic Processing area and Sterility Testing Area. Scope: This SOP is applicable for Personnel Qualification to work in Aseptic Processing and Sterility testing Area Procedure: For …
Read More »