Pharmazone- interviews for BD, GCP/GMP Services & Pharmacovigilance on 13th April 2024
Pharmazone Walk-in interviews on 13th April 2024 for BD, GCP/GMP Services & Pharmacovigilance
Greetings from Pharmazone!!
Walk-in interviews on 13th April 2024 for various positions in our organization.
Date: 13th April 2024
Time: 10:00 AM to 05:00 PM
Venue: 402, Shafalya Elegance Nr. Shakti Arcade, Science City Rd, Sola, Ahmedabad, Gujarat 380060
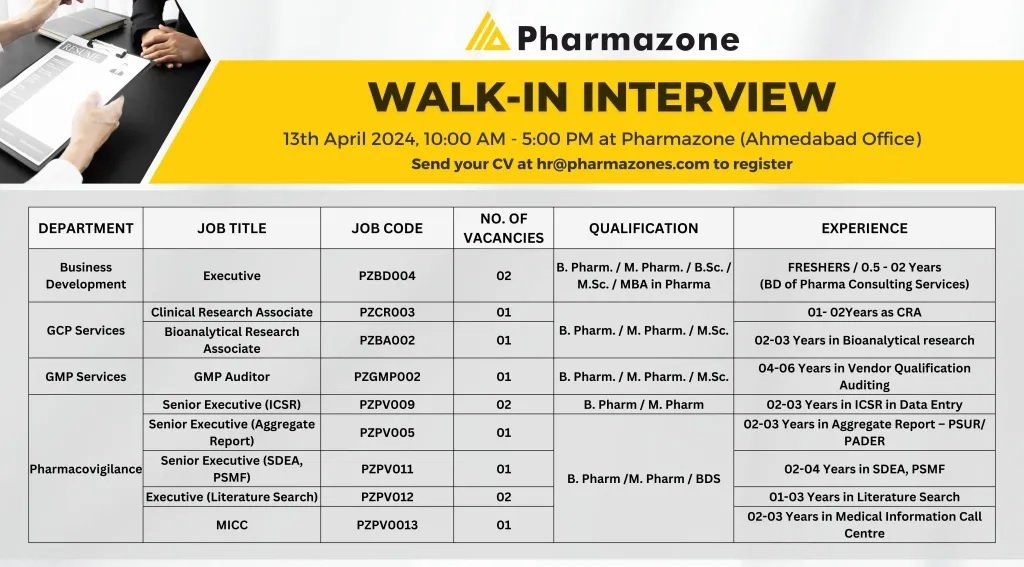
Department:Business Development
Executive
No. of vacancies: 02
Qualifications:B.Pharm/ M.Pharm/ B.sc./ M.sc/ MBA in Pharma
Experience-Freshers/ 0.5 – 2 Years (BD of Pharma Consulting Services)
GCP Services:
Clinical Research Associate
Bioanalytical Research Associate
Qualifications: B.Pharm/ M.Pharm/ M.sc
3) GMP Services
GMP Auditor
Qualifications: B.Pharm/ M.Pharm/ M.sc
04-06 Years in Vendor Qualification Auditing
Pharmacovigilance
Job Title: Senior Executive (ICSR), Senior Executive (Aggregate Report), Executive (Literature Search), MICC
Qualifications:B.Pharm/ M.Pharm/ BDS
No. of vacancies: 07
13th April 2024, 10:00 AM – 5:00 PM at Pharmazone (Ahmedabad Office)
Send your CV at hr@pharmazones.com to register