Dr. Reddy’s Laboratories- interview on14th April 2024@Hyderabad
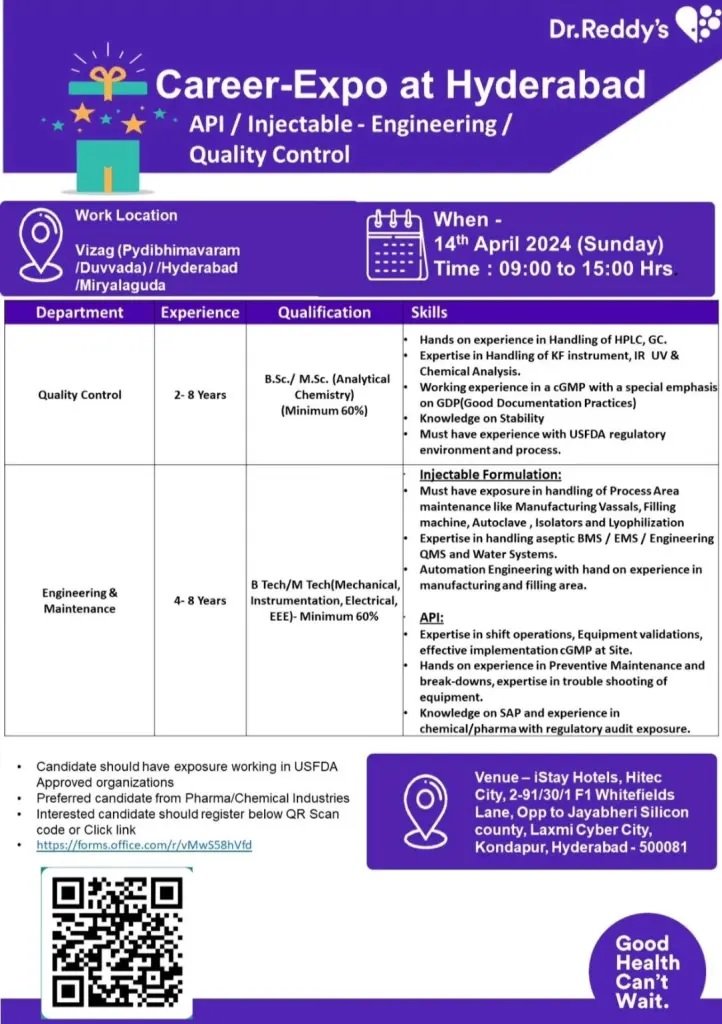
Dr. Reddy’s Laboratories walk in interview at Hyderabad on Sunday, 14th April 2024
We are pleased to announce an exciting opportunity at Dr. Reddy’s Laboratories
We are actively seeking talented individuals who are passionate about the pharmaceutical industry and excel in dynamic environments. If you possess these qualities, we encourage you to explore our open positions and take the next step in your career with us. Join our team and become a part of our innovative and driven workforce.
Career-Expo at Hyderabad
API / Injectable – Engineering/ Quality Control
Quality Control
2-8 Years (Experience)
Qualifications: B.Sc./ M.Sc. (Analytical Chemistry) (Minimum 60%)
Skills: Hands on experience in Handling of HPLC, GC.
Expertise in Handling of KF instrument, IR UV & Chemical Analysis.
Working experience in a cGMP with a special emphasis on GDP(Good Documentation Practices)
Knowledge on Stability
Must have experience with USFDA regulatory environment and process.
Engineering & Maintenance
Experience: 4-8 Years
Qualifications: B Tech/ M Tech(Mechanical, Instrumentation, Electrical, EEE)-Minimum 60%
Injectable Formulation:
Must have exposure in handling of Process Area
maintenance like Manufacturing Vassals, Filling machine, Autoclave, Isolators and Lyophilization Expertise in handling aseptic BMS/EMS/Engineering
QMS and Water Systems. Automation Engineering with hand on experience in manufacturing and filling area.
Knowledge on Stability
Must have experience with USFDA regulatory environment and process.
Venue- iStay Hotels, Hitec City, 2-91/30/1 F1 Whitefields Lane, Opp to Jayabheri Silicon county, Laxmi Cyber City, Kondapur, Hyderabad – 500081

