Emil Pharma-Walk in Drive for M.Pharm, B.Pharm, MSc in Production, QA, QC, Packing on 29th June 2025
Emil Pharmaceutical Industries Private Limited, founded by technocrats in 1989, is an established manufacturing and marketing firm focused on pharmaceutical and nutraceutical formulations for over 30+ years. Our company is headquartered in Mumbai, India, and has manufacturing units situated in Tarapur Industrial area, 100 km drive from Mumbai. Up to date equipments, outstanding quality structure and satisfying facilities are some key ingredients for success of Emil Pharmaceutical Industries. We meet WHO-GMP guideline and have been audited, approved and accredited by international authorities from Africa, South East Asia, Latin and CIS nations.
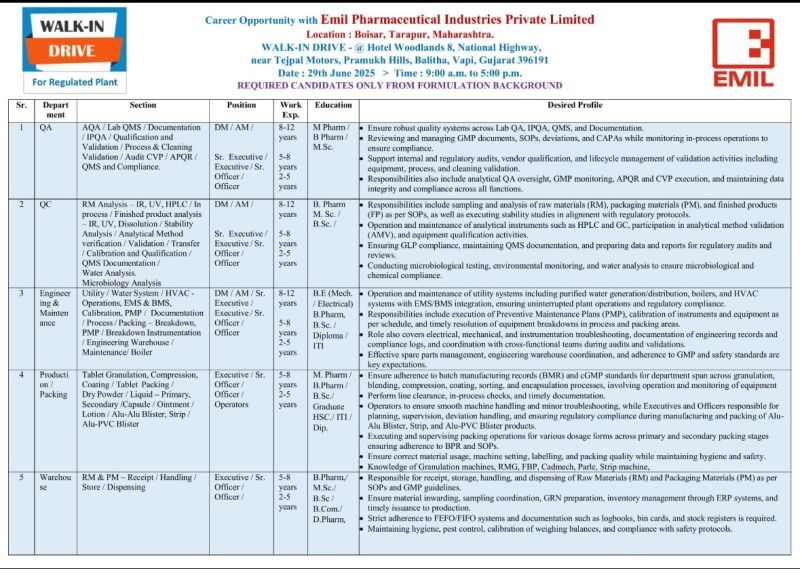
REQUIRED CANDIDATES ONLY FROM FORMULATION BACKGROUND
QA Department
Section : AQA / Lab QMS / Documentation IPQA / Qualification and Validation / Process & Cleaning Validation / Audit CVP/APQR / QMS and Compliance.
Post & Experience : DM/AM : 8-12 years ; Sr. Executive / Executive / Sr. Officer / Officer : 5-8 years ; 2-5 years
Qualification : MPharm / B Pharm / M.Sc.
Desired Profile : • Ensure robust quality systems across Lab QA, IPQA, QMS. and Documentation.
• Reviewing and managing GMP documents. SOPs, deviations, and CAPAs while monitoring in-process operations to ensure compliance.
• Support internal and regulatory audits, vendor qualification, and lifecycle management of validation activities including equipment, process, and cleaning validation.
• Responsibilities also include analytical QA oversight, GMP monitoring. APQR and CVP execution, and maintaining data integrity and compliance across all functions.
QC Department
Section : RM Analysis IR, UV, HPLC/ In process / Finished product analysis IR, UV, Dissolution / Stability Analysis / Analytical Method verification / Validation / Transfer / Calibration and Qualification / QMS Documentation / Water Analysis. Microbiology Analysis
Post & Experience : DM/AM/ Sr. Executive/ Executive / Sr. Officer / Officer ; 8-12 years 5-8 years 2-5 year
Qualification : B. Pharm M. Sc. / B.Sc.
Desired Profile : • Responsibilities include sampling and analysis of raw materials (RM), packaging materials (PM), and finished products (FP) as per SOPs, as well as executing stability studies in alignment with regulatory protocols.
• Operation and maintenance of analytical instruments such as HPLC’ and GC. participation in analytical method validation (AMV), and equipment qualification activities.
• Ensuring GLP compliance maintaining QMS documentation, and preparing data and reports for regulatory audits and reviews.• Conducting microbiological testing, environmental monitoring, and water analysis to ensure microbiological and chemical compliance.
Engineering & Maintenance Department
Section : Utility / Water System / HVAC – Operations, EMS& BMS. Calibration. PMP / Documentation / Process / Packing Breakdown. PMP Breakdown Instrumentation ‘ Engineering Warehouse / Maintenance/ Boiler
Post & Experience : DM / AM / Sr. Executive! Executive / Sr. Officer / Officer ; 8-12 years 5-8 years 2-5 years
Qualification : B.E (Mech. / Electrical) B.Pharm, B.Sc. / Diploma / ITI
Desired Profile : • Operation and maintenance of utility systems including purified water generation/distribution, boilers, and HVAC systems with EMS BMS integration, ensuring uninterrupted plant operations and regulatory compliance.
• Responsibilities include execution of Preventive Maintenance Plans (PMP), calibration of instruments and equipment as per schedule, and timely resolution of equipment breakdowns in process and packing areas.
• Role also covers electrical, mechanical, and instrumentation troubleshooting, documentation of engineering records and compliance logs, and coordination with cross-functional teams during audits and validations.
• Effective spare parts management, engineering warehouse coordination, and adherence to GMP and safety standards are key expectations.
Production / Packing Department
Section : Tablet Granulation. Compression, Coating / Tablet Packing / Dry Powder / Liquid – Primary. Secondary /Capsule / Ointment / Lotion / Alu-Alu Blister. Strip / Alu-PVC Blister
Post & Experience : Executive / Sr. Officer / Officer / Operators 5-8 years ; 2-5 years
Qualification : M. Pharm / B.Pharm / B.Sc./ Graduate HSC./ ITI / Dip.
Desired Profile :• Ensure adherence to batch manufacturing records (BMR) and cGMP standards for department span across granulation, blending, compression, coating, sorting, and encapsulation processes, involving operation and monitoring of equipment
• Perform line clearance, in-process checks, and timely documentation.
• Operators to ensure smooth machine handling and minor troubleshooting, while Executives and Officers responsible for planning, supervision, deviation handling, and ensuring regulatory compliance during manufacturing and packing of Alu- Alu Blister. Strip, and Alu-PVC Blister products.
• Executing and supervising packing operations for various dosage forms across primary and secondary packing stages ensuring adherence to BPR and SOPs.
• Ensure correct material usage, machine setting, labelling and packing quality while maintaining hygiene and safety.
• Know ledge of Granulation machines, RMG, FBP, Cadmech. Parle, Strip machine.
Warehouse Department
Section : RM & PM – Receipt • Handling / Store / Dispensing
Post & Experience : Executive / Sr. Officer / Officer : 5-8 years ; 2-5 years
Qualification : B.Pharm,/ M.Sc./ B.Sc/ B.Com./ D. Pharm,
Desired Profile : • Responsible for receipt, storage, handling, and dispensing of Raw Materials (RM) and Packaging Materials (PM) as per SOPs and GMP guidelines.
• Ensure material inwarding, sampling coordination. GRN preparation, inventory management through ERP systems, and timely issuance to production.
• Strict adherence to FEFO/FIFO systems and documentation such as logbooks, bin cards, and stock registers is required.
• Maintaining hygiene, pest control, calibration of weighing balances, and compliance with safety protocols.
Location : Boisar, Tarapur, Maharashtra.
Walk in Interview
Date : 29th June 2025
Time : 9:00 a.m. to 5:00 p.m
Venue : Hotel Woodlands 8, National Highway, near Tejpal Motors, Pramukh Hills, Balitha, Vapi, Gujarat 396191