HOLD TIME FOR DISPENSED MATERIAL, INTERMEDIATES AND FINISHED PRODUCTS
The purpose of this SOP is to describe the systematic procedure to establish the hold time of Dispensed materials, intermediate and bulk product prior to final packing. This SOP is applicable for Dispensed materials, intermediate as well as bulk product hold time study.
REFERENCE(S) & ATTACHMENT(S) of HOLD TIME STUDY
REFERENCE
WHO Technical Report Series No. 992, Annexure 4, 2015(General Guidance on Hold time studies)
CROSS REFERENCE DOCUMENTS
Quality Risk Management
SOP on Investigation
ATTACHMENTS
Attachment/Annexure/Formats
Attachment-I – Hold Time Study Sampling Register
DEFINITION & ABBREVIATION(S)
DEFINITION
Dispensed Material: A set of Dispensed active & excipients raw material as per approved formula comes under dispensed material.
Intermediate: Partly processed product that must undergo further manufacturing steps before it becomes a bulk product.
Bulk Product: Any pharmaceutical product that has completed all processing stages up to, but not including final packaging.
Hold Time Study: Hold-time studies establish the time limits for holding the materials at different stages of production to ensure that the quality of the product does not deteriorate significantly during the hold time.
Or
Hold time study shall be conducted to demonstrate that the bulk products and intermediates.
Retain the appropriate quality before processing to the next stage.
Meet the acceptance criteria and release specification for the finished products
ABBREVATIONS
BMR : Batch manufacturing record
MPS : Master Packing Specification
HDPE : High density Polyethylene
Ltd. : Limited
NA : Not Applicable
QA : Quality Assurance
QC : Quality Control
SOP : Standard operating Procedure
WHO : World health organization
RESPONSIBILITY
QA Department Person:
QA shall be responsible to collect the hold time samples as per hold time study Protocol/Datasheet and shall send duly filled “Sample request slip” to QC department.
QA shall be responsible to maintain hold time register and soft copy of hold day’s record.
QA shall be responsible to investigate in case of failure/discrepancies
QC Department Person:
QC shall be responsible to make entry of the sample as per defined procedure and analyze the sample as per defined procedure.
QC shall be responsible to share the report and investigate in case of failure/discrepancies.
Production Department Person:
Production shall be responsible to prepare the Test Requisition cum report and intimate to QA for sampling.
Quality Head and Plant head Responsible for:
Quality Head and Plant Head shall be responsible for review and approve the SOP.
Responsible for implementation of defined system.
PROCEDURE
SAFETY/PRECAUTION/EHS
Not applicable
Process flow chart
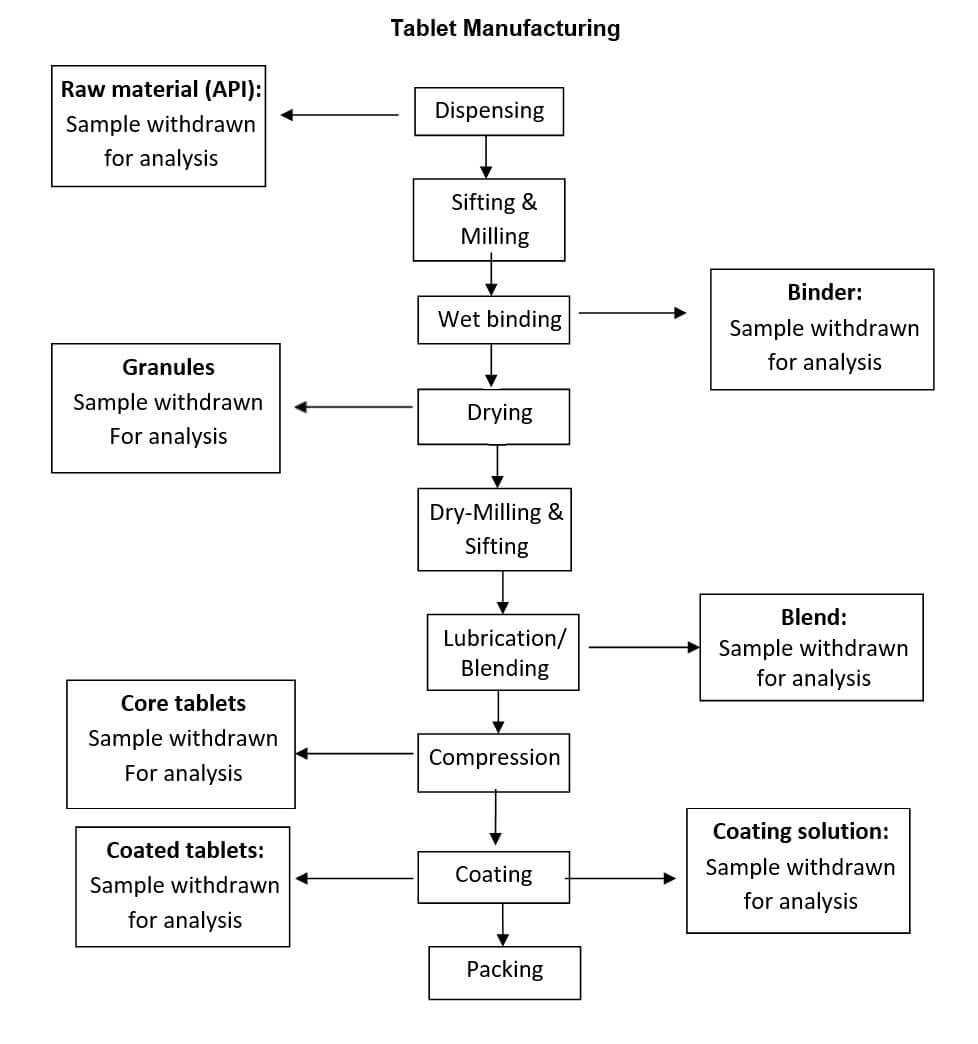
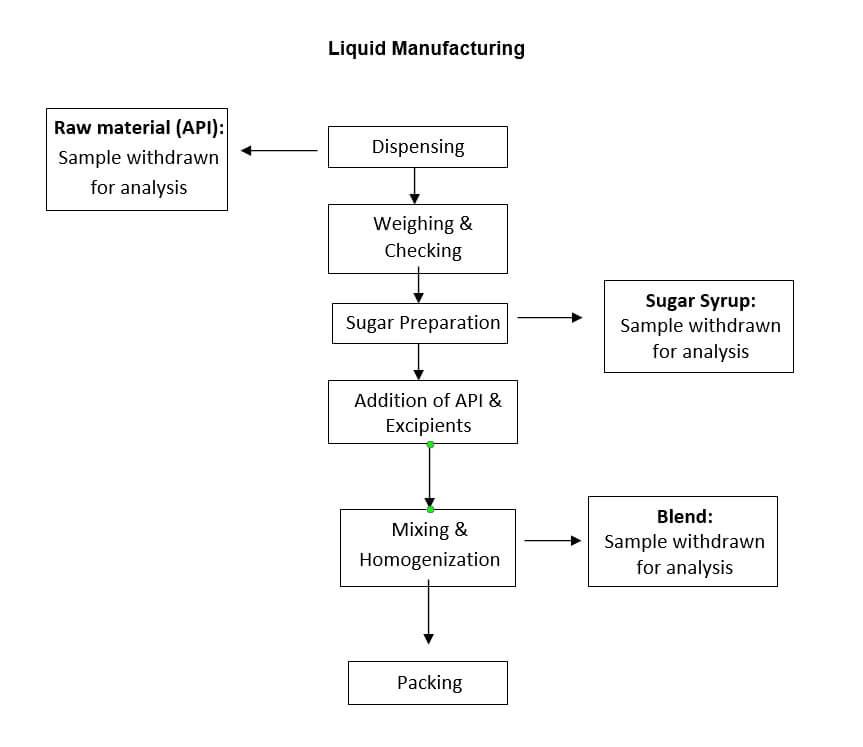
Hold time study shall be performed in one batch, which shall represent the respective product.
Hold Time study of Product shall be performed as per customize protocol.
Separate protocol shall be prepared and followed for different dosage forms and customize as per formulation.
Each protocol shall cover hold time study at different stages during manufacturing i.e. raw materials (dispensing), granulation fluid & granular blend (granulation), core tablet (compression), coating suspension & coated tablet (coating).
Storage condition of the product shall be mention in protocol as per dosage form for hold time study. The containers in which hold time samples are stored shall be the same pack (i.e. HDPE or S.S. container) as is used in production. Reducing the size of container, when this is necessary for testing holding time, shall be justified.
The environmental conditions for sample storage shall be the same as those of quarantine area/manufacturing stage and mentioned in the respective protocol.
A sampling plan shall be established and defined in the protocol for taking samples for testing at different intervals.
The amount of sample required shall be calculated based on the interval, test required in respective product/material specification or analytical test procedure and quantity will be mentioned in respective protocol sampling plan.
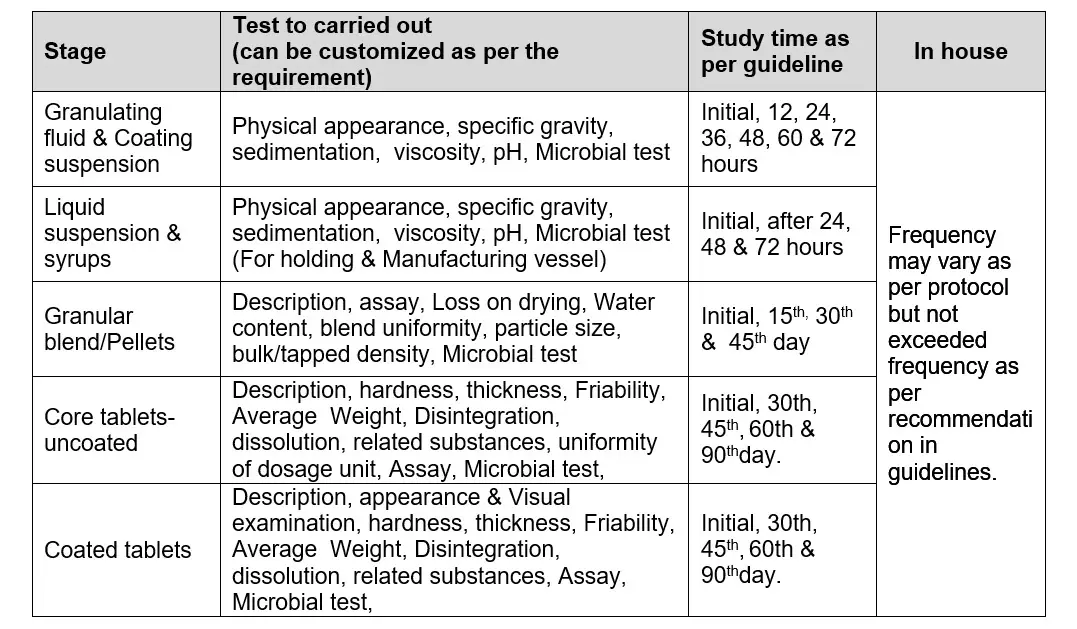
Hold time study shall be performed in following case:
Change in storage condition
Change in formulation like addition or deletion of ingredients.
Change in manufacturing process like change in granulation method etc.
For new formulation.
Change in API Source.
The hold time study for tablet, liquid and Dry Powder shall be performed up to :
For blend, granulating fluid, coating suspension & tablet:
Hold granulating fluid/coating suspension for 72 hours (samples send to QC at initial, end of 24 & 48 hours).
Hold blend for 45 days (sample send to QC at Initial, 15th 30th & 45thday)
Hold core tablet for 90 days (sample send to QC at Initial, 30th, 45th, 60th & 90th)
Hold Coated tablets for 90 days (sample send to QC at Initial, 30th, 45th, 60th & 90thday).
Hold time samples (Granulating fluid, Blends, Core tablets, Coated tablets & Coating Suspensions) handling during tablet manufacturing:
Raw Materials:
QA person shall collect the required quantity of sample of starting materials as per the protocol and stored in the HDPE container in quarantine.
The entry of hold time samples collected should be made in the hold time sampling register as per Attachment-I
On completion of hold time, ‘Test Requisition Cum Report’ should be filled by QA and send to QC along with the sample.
Granulating Fluid:
QA person shall collect the required quantity of sample of granulating fluid (binder) as per the protocol and stored in the S.S. container in controlled area.
The entry of hold time samples collected should be made in the hold time sampling register.
On completion of hold time, ‘Test Requisition Cum Report’ should be filled by QA and send to QC along with the sample.
Blend Stage:
QA person shall collect the required quantity of the sample of blend/granules/pellets as per the protocol or as specified in BMR/MPS and stored in the HDPE container in blend Quarantine.
The entry of hold time samples collected should be made in the hold time sampling register.
On completion of hold time, ‘Test Requisition Cum Report’ should be filled by QA and send to QC along with the sample.
Compression Stage:
QA person shall collect the required quantity of the sample of core tablet as per the protocol and stored in the HDPE container in tablet Quarantine.
The entry of hold time samples collected shall be made in the hold time sampling register.
On completion of hold time, ‘Test Requisition Cum Report’ shall be filled by QA and send to QC along with the sample.
Coating Stage:
QA person shall collect the required quantity of the sample of coated tablet as per the protocol and stored in the HDPE container in tablet Quarantine.
The entry of hold time samples collected should be made in the hold time sampling register.
On completion of hold time, ‘Test Requisition Cum Report’ should be filled by QA and send to QC along with the sample.
Coating Suspension:
QA person shall collect the required quantity of coating suspension as per the protocol and stored in the S.S container in the tablet Quarantine.
The entry of hold time samples collected should be made in the hold time sampling register.
On completion of hold time, ‘Test Requisition Cum Report’ should be filled by QA and send to QC along with the sample.
Hold time (Tablets/sachets/bottle) handling at packing stage :
For Tablets/sachets/bottle/pouch which kept in factory premises at specified storage condition beyond the hold time data specified, sample shall be send to QC for analysis before release of batch.
Head QA/Quality head shall recommend the testing parameter for re-testing, Preferably stability indicating test shall be consider for retesting (Assay, Average weight, Fill weight, Related Substances (impurities) dissolution etc.).
“Test requisition cum report” slip shall be prepared by packing officer and shall be forwarded to IPQA officer for collection of sample.
For chemical testing 50 units for tablet/capsule and for bottle/sachets 50 gm or as per QC requirement.
For microbiology testing required 10 gm. or as per microbial requirement from each dosages form separately shall be send to QC.
Certificate of analysis of Hold time stage receive from QC department shall be attached with respective BMR/BPR and hold time shall be updated in soft copy for future reference soft copy shall be password protected by concerned QA officer.
Batch will be transferred only after ensuring the compliance of QC result.
If hold time sampling skip or forget at defined frequency, then raise QMS & justify the reason then Sampling shall be done in next hold time frequency to be analyzed.
If any finished/ semi-finished product crosses its define hold time frequency then raise QMS Document for justification, semi-finished/Finished product shall be retest before proceeding to next stage or final decision shall be taken by QA Head.
Hold time sample shall be withdrawn in next working day in case of holiday.
In case a batch /product in retested and result found the complying then the existing hold time shall be updated accordingly for the future reference.
REVISION HISTORY – New SOP Prepared.

