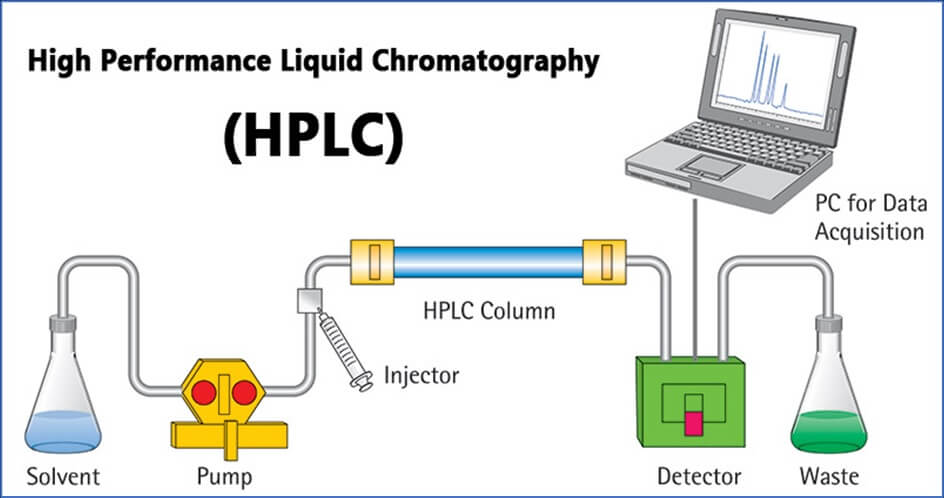
Procedure for calibration of HPLC (WATERS ALLIANCES)
In the pharmaceutical industry, where precision and accuracy are non-negotiable, High-Performance Liquid Chromatography stands as a stalwart technology. From drug development to quality control and regulatory compliance, HPLC plays a vital role in ensuring the safety, efficacy, and consistency of pharmaceutical products. As technology continues to advance, HPLC will undoubtedly remain at the forefront of analytical techniques, contributing to the advancement of pharmaceutical science and the well-being of patients worldwide.
HPLC CALIBRATION OBJECTIVE
To lay down the procedure for calibration of the HPLC (Waters Alliances 2695, Separation Modules).
To ensure that the calibration procedure of HPLC meets the acceptance criteria. This SOP shall be applicable for the HPLC system. (WATERS Alliances 2695 Separation Modules).
RESPONSIBILITY
Quality Control Executive/Officer.
ACCOUNTABILITY
Quality Control Manager.
PROCEDURE FOR CLEANING
Check that the power supply to the instrument is switched ‘ OFF ‘ before cleaning. Clean the instrument with a clean dry cloth every day. Occasionally wet cloth dipped in dilute soap solution may be used. Precaution has to be taken to clean the instrument immediately with dry cloth to remove the moisture.
PROCEDURE FOR CALIBRATION:
To perform the calibration of the instrument as per procedure given below, follow the operating instruction given in the SOP of the instrument.
Following parameters shall be calibrated:
For PUMP:
Flow rate Accuracy
For Gradient Performance verification:
For Detector:
Wave length accuracy
Linearity Response of Detector.
For Auto Sampler:
Injection Repeatability & Percentage carryover.
Linearity of injection volume.
For Column Oven:
Oven Temperature.
For Pump calibration:
1. Flow rate Accuracy:
Select the water as mobile phase and temperature at 25°C.
Put the suction filter in to the reservoir containing the water as mobile phase.
Set the flow rate at 2.0 ml per minute and open the By-pass system and start purging to remove air bubble from the tubing’s. Decrease the flow rate slowly and stop the pump.
Connect the union between pump outlet and detector inlet.
Collect the mobile phase at the detector outlet into a cleaned dried 25ml measuring cylinder (previously weight) for known time i.e. at 0.5ml/min for 10 min and calculate the flow rate /min by given formula.
Vol. Dispense in the selected time =
(Wt of filled Measuring Cylinder(W2) – Wt of empty Measuring Cylinder (W1) ) / (Wt /ml of water at 25°C )
Flow rate per min = Volume dispense in selected time / Time.
Repeat the above procedure for Flow rate of 1.0ml, 1.5ml and 2.0ml for 5 minutes.
Record the observation as per given in Annexure 1.
2. For Gradient Performance verification:
Connect the union between pump outlet and detector inlet.
Place the suction filter assembly of gradient line A & B in to the solvent reservoir containing methanol (100%).
Place the suction filter assembly of gradient line C & D in to the solvent reservoir containing 5.6mg/Lit. solution of Propyl paraben in methanol.
Prime the pump.
Fill the gradient table parameter as mention below:
| Time | Flow | %A | %B | %C | %D | Curve |
| Initial | 2.0 | 50 | 50 | 0 | 0 | |
| 2.0 | 2.0 | 0 | 0 | 50 | 50 | 11 |
| 6.0 | 2.0 | 50 | 50 | 0 | 0 | 11 |
| 10.0 | 2.0 | 45 | 45 | 10 | 0 | 11 |
| 12.0 | 2.0 | 50 | 50 | 0 | 0 | 11 |
| 14.0 | 2.0 | 45 | 45 | 0 | 10 | 11 |
| 16.0 | 2.0 | 50 | 50 | 0 | 0 | 11 |
| 18.0 | 0.0 | 50 | 50 | 0 | 0 | 11 |
Set the detector wavelength to 254 nm.
Save the method set as GPV_ MS.
Set run time for 18 minutes and Inject 20ml methanol as a sample.
Check the obtained chromatogram for gradient steps.
Process the GPV run with a processing method, to obtain the % of the full scale Peak.
Save the result and generate a repot method that gives the GPV %.
%GPV should be 10% ±0.5.
FOR AUTO SAMPLER:
1. Injection Repeatability & % Carryover:
Chromatographic condition:
Wave length: 254 nm
Mobile phase: Methanol: water (85:15) (isocratic)
Flow rate: 1ml /min.
Test Sample: Naphthalene (26mg/100ml in methanol)
Injection Volume: 10µl.
Column: symmetry C18 5µ: 150 X 4.6 mm
Run the system as per the above chromatographic Condition. Wait until the base line stabilizes. Prepare a sample set method for the following injections
10µl methanol as a blank.
10µl test in 6 replicates
10µl methanol as a blank for carryover
Process the acquired data and calculate the % RSD for Retention time & Peak area.
Calculate the % carry over (area of sample in blank for carry over/ average area of sample in test replicates x100).
Record the observation as per given in Annexure I.
2. Linearity of Injection Volume:
Run the system as per the above chromatographic Condition given in step 8.1.1. Wait until the base line stabilizes, than inject 10µl methanol as a blank.
Inject 10µl, 20µl, 30µl 40µl and 50µl. of test sample in duplicate.
Record the observation as per given in Annexure I. Plot the graph of Injection volume vs Response (Peak area).
For Detector:
1. Wave length Accuracy:
Chromatographic condition:
Wave length: 250nm to 266nm at the interval of 2nm.
Mobile phase: Methanol
Flow rate: 1ml /min.
Test Sample: Uracil (20 ppm in methanol).
Injection Volume: 20µl.
Column: symmetry C18 5µ: 150 X 4.6 mm.
Run the system as per the above chromatographic Condition, Wait until the base line stabilizes, then inject 20µl methanol as a blank.
Inject 20µl of test sample in duplicate at the wavelength of 250 nm.
Repeat the process at the wavelength of 252 nm to 266 nm at the interval of 2 nm.
Process and save the acquired data and record the response at each wavelength in calibration table of Annexure I.
Compare the results and measure the wavelength maximum in terms of maximum response of area.
2.Linearity of Detector Response.
Prepare the test sample of Uracil 2 ppm to 10 ppm at the interval of 2 ppm in methanol.
Run the system as per the chromatographic condition given in step 9.1.1, Wait until the base line stabilizes, then inject 20µl methanol as a blank.
Separately inject each 2 ppm to 10 ppm solution induplicate and take the mean of the solution for linearity study.
Plot the graph for Concentration VS Response (Peak area) and calculate the Coefficient of regression.
Record the observation as per given in Annexure I.
For Column Oven:
Oven Temperature:
Set the column oven to 40°C.
Place the probe of thermometer inside the column oven.
Note down the initial temperature displayed on the instrument.
Note down the temperature displayed by the probe.
Allow the column oven to stand for 30min. and note down the temp.
Repeat the above procedure similarly for Temperature 50°C and 60°C.
Record the instrument displayed temperature and thermometer reading in annexure –I.
Enter the details in the respective instrument log book.
Acceptances criteria:
| MODULE | PARAMETER | STANDARD | TOLERANCES | FREQUENCY |
| PUMP | I) Flow rate Accuracy | By measurement of dispensed volume in specified time. | ±2% of Set flow. | QUATERLY |
| II) For Gradient Performances Verification: | % Height at each step. | 10% ±0.5% | QUATERLY | |
| Detector | I) Wave length accuracy | Maximum response of Uracil at different wavelength. | 258nm ±2nm | QUATERLY |
| II) Linearity Response of Detector. | Response of Uracil at different concentration 2-ppm to 10 ppm. | Regression Coefficient NLT 0.99 | QUATERLY | |
| Auto Sampler | I) Injection Repeatability & Percentage carryover. | By 26 mg / 100 ml Naphthalene in methanol. | RSD NMT 0.5% & % Carryover NMT 0.01% | QUATERLY |
| II) Linearity of injection volume. | By different injection volume of same concentration. | Regression Coefficient NLT 0.99 | QUATERLY | |
| Column Oven
|
Oven Temperature. | By calibrated thermometer at different temperature i.e. 40°C, 50°C, 60°C. | ± 2°C at Set temperature. | QUATERLY |
| ± 0.5°C between thermometer reading and instrument displayed value. |
Forms and Records (Annexures)
CALIBRATION OF HPLC – WATERS [ALLIANCE] – Annexure-1
Distribution
Master copy – Quality Assurance
Controlled copies- Quality Assurance, Production, Quality Control. engineering
History
Annexure-1
CALIBRATION OF HPLC – WATERS [ALLIANCE]
QUALITY ASSURANCE DEPARTMENT
INSTRUMENT CALIBRATION FORMAT – HIGH-PERFORMANCE LIQUID CHROMATOGRAPH
| LOCATION | CALIBRATION PROCEDURE NO. | ||
| MANUFACTURED BY | INSTRUMENT ID. NO. | ||
| MODEL NO. | EFFECTIVE DATE |
CLEANLINESS OF EQUIPMENT / INSTRUMENT BEFORE CALIBRATION: SATISFACTORY/ NOT SATISFACTORY
MODULE: PUMP
- FLOW RATE ACCURACY:
STOP WATCH USED:
| Flow rate set ml/min (F) | Time set
Min (T) |
Wt of empty Measuring cylinder
(W1) g |
Wt of filled Measuring cylinder
(W2) g |
Vol. dispense (V)
V = (W2-W1) / Wt per ml of water At 25°C |
Flow per min
= V / T |
Tolerance
±0.2 % |
| 0.5 | 10 | 0.49 – 0.51 | ||||
| 1.0 | 5 | 0.98 – 1.02 | ||||
| 1.5 | 5 | 1.47 – 1.53 | ||||
| 2.0 | 5 | 1.96 – 2.04 |
STATUS: PASS/ FAIL
2. Gradient Performances Verification:
| S.NO. | Peak name | Height | Acceptance Criteria |
| 1. | Full scale | 10 %± 0.5 | |
| 2. | A / B / C | ||
| 3. | A / B / D |
STATUS: PASS/ FAIL
MODULE: DETECTOR
1.Wave Length Accuracy:
Standard Uracil used (B. No. / Lot No): _______________________
Standard dilution:
| Wave length
(nm) |
Replicate Injection | RT of Uracil | Observation | Wavelength maximum observed (nm) | Acceptances Criteria | |
| Peak Area | Mean | |||||
| 250 | 1 | 258 ± 2 nm | ||||
| 2 | ||||||
| 252 | 1 | |||||
| 2 | ||||||
| 254 | 1 | |||||
| 2 | ||||||
| 256 | 1 | |||||
| 2 | ||||||
| 258 | 1 | |||||
| 2 | ||||||
| 260 | 1 | |||||
| 2 | ||||||
| 262 | 1 | |||||
| 2 | ||||||
| 264 | 1 | |||||
| 2 | ||||||
| 266 | 1 | |||||
| 2 | ||||||
STATUS: PASS/ FAIL
- Linearity Responses of Detector:
Standard Uracil used (B. No. / Lot No):_______________________
Standard dilution:
| Conc. of Uracil
(ppm) |
Replicate Injection | RT of Uracil | Observation | Correlation coefficient | Acceptances Criteria | |
| Peak Area | Mean | |||||
| 2 | 1 | r ≥0.99 | ||||
| 2 | ||||||
| 4 | 1 | |||||
| 2 | ||||||
| 6 | 1 | |||||
| 2 | ||||||
| 8 | 1 | |||||
| 2 | ||||||
| 10 | 1 | |||||
| 2 | ||||||
STATUS: PASS/ FAIL
MODULE: AUTO SAMPLER
- Injection Repeatability and % Carry Over:
Standard Naphthalene used (B. No. / Lot No):_______________________
Standard dilution:
% Carry over = Area of sample in blank/ Mean Area of sample in tests X 100
| Replicate injection | RT of Naphthalene | Peak Area | Acceptances Criteria | % Carry Over | Acceptances Criteria |
| 1 | %RSD of RT
is NMT 0.5% & % RSD of Area is NMT 1.0%
|
NMT 0.01% | |||
| 2 | |||||
| 3 | |||||
| 4 | |||||
| 5 | |||||
| 6 | |||||
| Mean | |||||
| % RSD | |||||
| % Carry over blank |
STATUS: PASS/ FAIL
- Linearity of Injection Volume:
| Injection Volume | 10.0 µl | 20.0 µl | 30.0 µl | 40.0 µl | 50.0 µl |
| Area of injection 1 | |||||
| Area of injection 2 | |||||
| Mean area | |||||
| Correlation coefficient | Acceptance Criteria | r ≥0.99 | |||
STATUS: PASS/ FAIL
MODULE: COLUMN OVEN
- Oven Temperature:
Thermometer Id.No. _______________________
| S No. | Set Temperature
(°C) |
Observed Temperature (°C) | Difference | Acceptances Criteria | |
| Instrument | Thermometer | ||||
| 1 | 40 | ± 2°C at each set temperature
& ± 0.5° C between instrument and thermometer readings |
|||
| 2 | 50 | ||||
| 3 | 60 | ||||
STATUS: PASS/ FAIL
OPINION: INSTRUMENT IS SUITABLE/ NOT SUITABLE FOR USE.
NEXT CALIBRATION DUE ON: ————————————————————–
Calibrated by: Checked by: Approved by:
Date : Date : Date :
HPLC Chromatography troubleshooting
For More Pharma Updates Visit –https://pharmaguidances.com