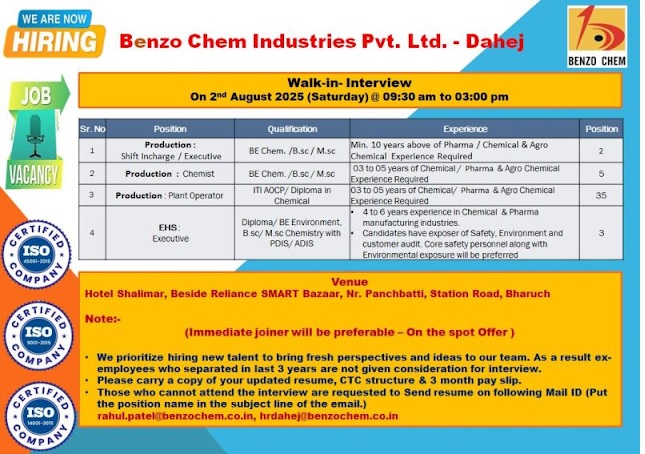
Benzo Chem Industries -Walk In Interviews For Production & EHS On 2nd August 2025 @Bharuch
Production Department
1. Production Shift Incharge / Executive ◾️Qualification: BE Chemical / B.Sc / M.Sc
◾️Experience: Minimum 10 years in Pharma/Chemical/Agro industries ◾️Vacancies: 2
2. Production Chemist
◾️Qualification: BE Chemical / B.Sc / M.Sc
◾️Experience: 3 to 5 years in Chemical/Pharma/Agro industries ◾️Vacancies: 5
3. Production Plant Operator
◾️Qualification: ITI AOCP / Diploma in Chemical
◾️Experience: 3 to 5 years in Chemical/Pharma/Agro industries ◾️Vacancies: 35
4. EHS Executive
◾️Qualification: Diploma / BE Environment / B.Sc / M.Sc with PDIS or ADIS
◾️Experience: 4 to 6 years in Chemical/Pharma manufacturing, safety, environment, audits
◾️Vacancies: 3
Date : 2nd August 2025, Saturday
🕒 Time : 09:30 AM to 03:00 PM
📍 Venue : Hotel Shalimar, Beside Reliance SMART Bazaar, Nr. Panchbatti, Station Road, Bharuch