In-process control of oral drug product during manufacturing & Packing
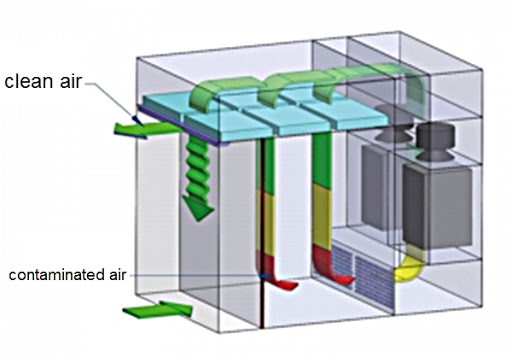
Objective To lay down the procedure for in-process control of oral drug products during manufacturing & Packing. Scope This procedure is applicable for in-process sampling, analysis, and reporting to be carried out during manufacturing and packing of drug products at the formulation Plant. Responsibility Quality Assurance and production personnel shall responsible to follow the procedure…
Read More “In-process control of oral drug product during manufacturing & Packing” »