QUALIFICATION STUDY FOR VISUAL INSPECTOR
To establish documented evidence that Visual Qualification Protocol is capable to qualify the visual inspector, within the specified quality attributes thereby establishing the Visual Inspectors qualification procedure.
Detect and remove units of drug product with predefined defects in a reproducible manner in a controlled process.
Each final container of all preparations form must be inspected to the fullest extent possible for the presence of observable foreign and particulate matter and other predefined defects.
TABLE OF CONTENTS VISUAL INSPECTOR STUDY PROTOCOL
- Cover Page
- Table of content
- Pre-Approval
- Objective
- Scope
- Responsibilities
- Training
- Classification of rejections
- Requalification Criteria
- Documentation
- Summary & Conclusion
- Enclosed documents
- Abbreviations
PRE-APPROVAL
This document is prepared by the validation team Under the authority of their QA Manager. Hence, this document before being effective shall be approved.
OBJECTIVE
This study protocol is designed to establish documented evidence that Visual Qualification is capable to qualify the visual inspector, within the specified quality attributes thereby establishing the Visual Inspectors qualification procedure.
Detect and remove units of drug product with predefined defects in a reproducible manner in a controlled process. Each final container of all preparations form must be inspected to the fullest extent possible for the presence of observable foreign and particulate matter and other predefined defects.
Scope
The scope of this study is to describe the procedure for different tests, acceptance criteria, Re-qualification criteria and documentation to be used in qualification of Visual Inspector.
Final visual inspection is performed manually by human operators.
Prior to being qualified as an inspector, each inspector must be successfully trained and qualified as per protocol procedure.
This protocol shall be applicable to all the Visual Inspectors
Responsibilities
The responsibilities of different departments for the Qualification of Visual Inspector as below.
Quality Assurance
QA shall prepare & review the protocol and report.
QA shall evaluate deviations & change controls.
Production
Review of Protocol and report and to assist in the protocol preparation and execution.
QA Head
QA Head shall approve the protocol and report.
QA Head shall approve deviations & change controls.
QA Head shall ensure implementation of the protocol.
HR Departments
HR department shall review the protocol and report.
HR department shall be arranging the facility for medical checkup.
TRAINING
Each trainee shall be followed by training programmed for visual inspections and the training record shall be maintained
CLASSIFICATION OF REJECTIONS
For W.F.I section
- Neck Problem
- Black/Surface/Solution Particle
- Twist Problem
- Less Volume, High Volume
- Extra Plastic
- Cavity No. and its Clarity
- Dirty Ampoule, Rough Surface
- Empty Ampoule, Deep Line
For Oral Liquid section
- Foreign /Black particles, Fiber material in bottle.
- Sealing of caps.
- Cut or center out of Caps.
- Volume Variation.
- Deform shape etc.
For Oral Solid Dosage Form section.
- Foreign product, foreign material.
- Tablet not in uniform size.
- Missing debossing.
- Abnormal discoloration of products.
- Large dark staining on product.
- Blooming, Bridging.
- Color Variation.
- Orange Peel/Roughness.
- Twinning
- Blushing
- Cracking/Splitting.
- Peel Off.
- Shade variation, Infilling.
REQUALIFICATION CRITERIA
Requalification of visual inspectors shall be done in case of following reasons:
- Any major type of rejection/abnormalities found incidentally.
- Any major change in visual inspection booth.
- Change in qualification procedure.
Frequency: Six Month ± one month (For SWFI section).
Frequency: Yearly ± one month (For Oral Liquid and tablet section)
DOCUMENTATION
Results and reports shall be compiled in a binder. Binder shall contain the following sequentially.
- Summary Report
- Requalification Protocol
- Test Reports and Certificates
Summary report shall be in narrative form, which describes the work as well as conclusion / certification regarding acceptability. Summary Report shall contain the following:
- Approval
- Objective
- Acceptance criteria
- Brief validation methodology
- Evaluation of results
- Final result summary
- Deviation, failure investigation reports and corrective actions (if any)
- Conclusion
SUMARRY & CONCLUSION
Based on the review of results a conclusion shall be drawn and documented in the summary report. Conclusion shall be a clear statement of compliance or non-compliance of the Visual Inspector Qualification with the acceptance criteria of the performance qualification protocol.
Datasheet #1 Medical Checkup of Eye Record
| Sr. No. | Name | Eye Vision | Colour Blindness | Report Attached | Remark |
Prepared By (Date/sign) Approved By(Date/sign)
Datasheet #2 Qualification Kit for Visual Inspection
Quantity of Ampoules for Qualification: 100
True Positive Ampoules: 20 Ampoules, True Negative Ampoules: 80 Ampoules
| Sample Code | Type of Rejection | Ampoule No. |
Prepared By (Date/sign) Approved By(Date/sign)
Datasheet #3 Visual inspection record document for SVP section
VISUAL CONDITIONS:
- 2,000 – 3,750 Lux (NLT 2000 Lux) for colored ampoules or plastic container.
- Black and white backgrounds.
- At least 5 seconds viewing against each background.
TYPE OF REJECTIONS:
- Neck Problem
- Black/Solution/Surface Particle
- Twist Problem
- Rough Surface
- Less Volume
- Extra Plastic
- High Volume
- Cavity No. and its Clarity
- Dirty Ampoule
- Empty Ampoule
- Deep Line
SELECTION OF VISUAL INSPECTOR-
- All visual inspectors had to gone through the eye medical checkup in order to assess visual acuity.
- Color blindness of each personnel shall be verified.
- Each trainee shall be followed by training programmed for visual inspections.
- All visual inspectors had to gone through the visual inspection kit in order to assess visual acuity.
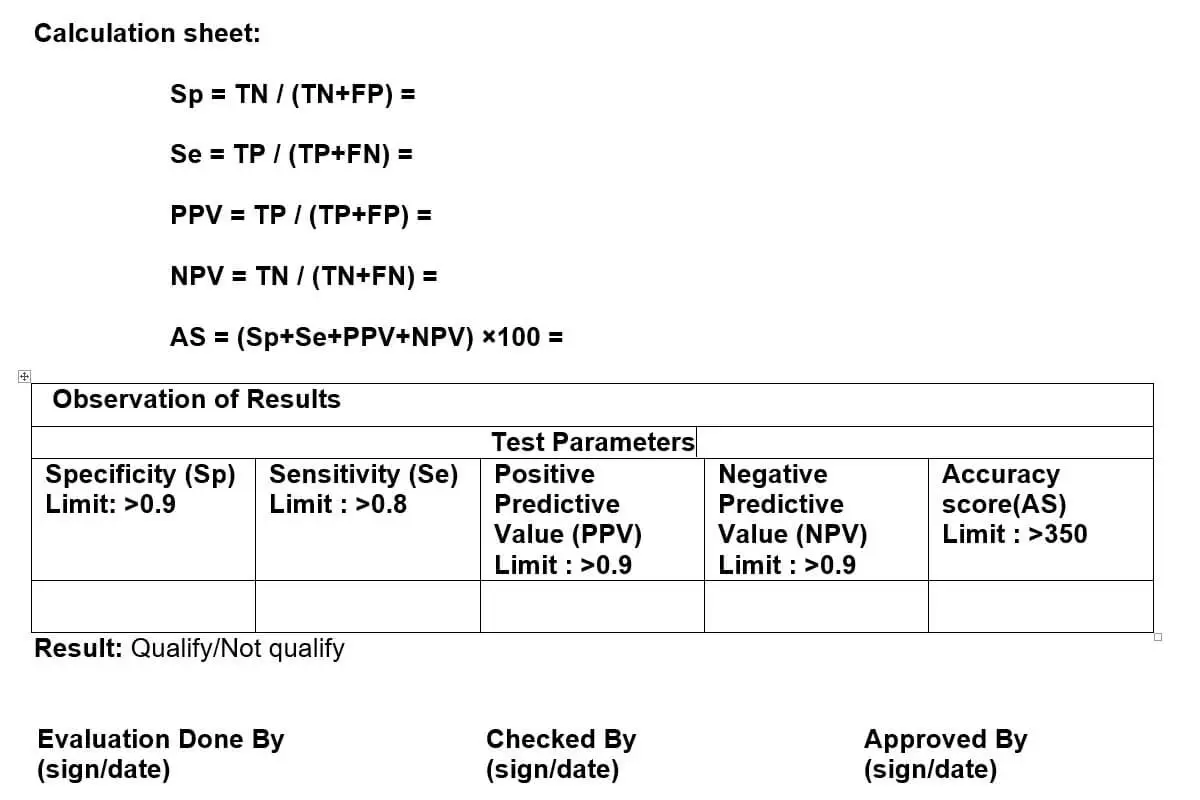
CALCULATION OF RELIABILITY:
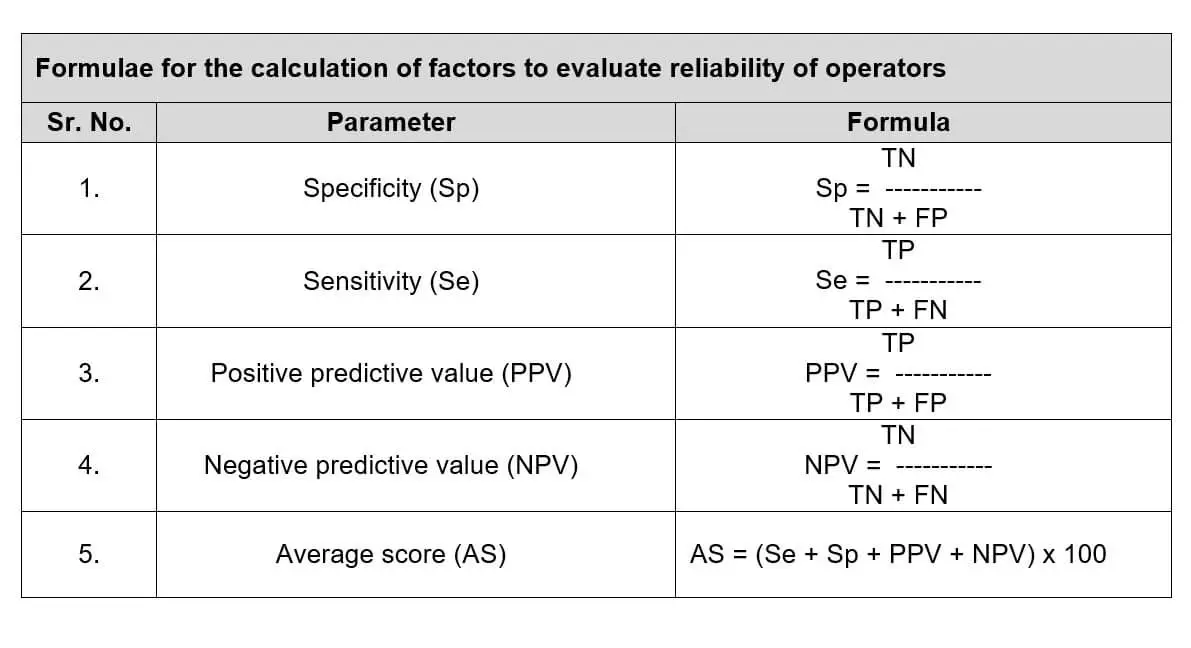
- Sensitivity (Se): representing the detection of non-conformity.
- Specificity (Sp): representing only the detection of conforming vials.
- Positive predictive value (PPV): being the probability that a detected non-
conformity is true. - Negative predictive value (NPV): being the probability that a conformity result is true.
- True Positive (TP): 20 Positive (Defective Ampoule) ampoule of the validation
- True Negative (TN): 80 Negative (good Ampoule) ampoule of the validation kit.
- False Positive (FP): Good Ampoule counted as defective by the visual.
- False Negative (FN): Defective Ampoule counted as good by the visual
- On the basis of these four factors (Sp, Se, PPV and NPV) evaluate the accuracy score of inspector.
- Inspectors who get the scores mentioned in above table will qualify the test and are able to do the visual inspection.
- In-process Quality Assurance person shall observe and verify the whole Qualification activity.
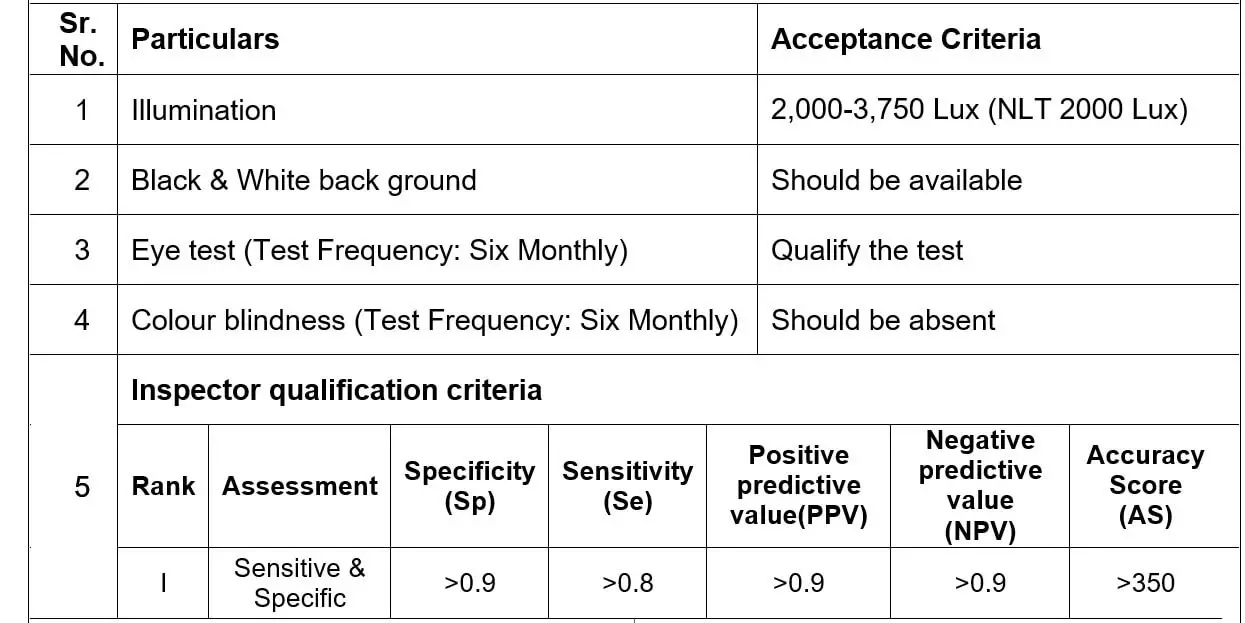
ACCEPTANCE CRITERIA
PROCEDURE:
Visual inspection of ampoules is carried out by trained employee/person under visual inspection booth. Ampoules are visually inspected for various kinds of possible abnormalities as define above. All visual inspector shall be qualified before induction in visual inspection as per following procedure:
Preparation of ampoules for qualification:
Select 20 positive and 80 negative ampoules. Negative and positive units were chosen from either from same batch or from different batches which covers all the types of rejection define above. Different qualification ampoules shall be prepared.
A defects album shall be prepared and Training shall be performed to all selected Inspectors. All selected inspectors should be passed in eye test and colour blindness by using the above test samples and defects album.
Qualification shall be performed by using the above test samples. Keep the entire (positive and negative) ampoules in box / crates for individual inspector.
Gently swirl or invert each individual container, making sure that no air bubbles are introduced.
Observe for 5 seconds in front of the white panel. Repeat in front of the black panel.
Keep the rejected ampoules in different crates after visual Inspection.
After completion of visual inspection of ampoules, stop the activity and inspect rejected ampoules and find out whether all the known rejection recovered during checking or not.
If all the known rejected ampoules/vials, ampoules recovered/qualify the acceptance criteria during visual inspection that means that the visual inspector is fit for visual inspection process.
Note: Put (√) if ampoule are good. Mention the code shown below for different type of defect.
| Code | Type of Rejection | Code | Type of Rejection | Code | Type of Rejection |
| A | Neck Problem | E | Less Volume | I | Dirty Ampoule |
| B | Black Particle | F | Extra Plastic | J | Empty Ampoule |
| C | Twist Problem | G | High Volume | K | Deep Line |
| D | Rough Surface | H | Cavity No. and its Clarity |
Evaluation Table
| Parameters | Description | Ampoule Number | Observation Number |
| True Positive (TP) | Total defective Ampoules | 20 | Total Ampoule of Visual Inspection kit = 100 |
| True Negative (TN) | Total good Ampoules | 80 | |
| False Positive (FP) | Good Ampoule counted as defective | ||
| False Negative (FN) | Defective Ampoule counted as good |
Datasheet #4 Visual inspection record document for oral liquid dosage
VISUAL CONDITIONS:
- NLT 1000 Lux.
- Black and white backgrounds.
- At least 5 seconds viewing against each background.
TYPE OF REJECTIONS:
- Dent Problem
- Black/ White Particle
- Rough Surface
- Less Volume
- Extra Plastic
- High Volume
- Dirty Bottle
- Empty Bottle
- Deep Line/ Deep Scratch Over Bottle And Screw Cap.
SELECTION OF VISUAL INSPECTOR-
- All visual inspectors had to gone through the eye medical checkup in order to assess visual acuity.
- Color blindness of each personnel shall be verified.
- Each trainee shall be followed by training programmed for visual inspections.
- All visual inspectors had to gone through the visual inspection kit in order to assess visual acuity.
- True Positive (TP): 20 Positive (Defective Bottle) ampoule of the validation
- True Negative (TN): 80 Negative (Good Bottle) ampoule of the validation kit.
- False Positive (FP): Good Bottle counted as defective by the visual
- False Negative (FN): Defective Bottle counted as good by the visual
- On the basis of these two factors (FP, FN) evaluate the accuracy score of inspector.
- Inspectors who get the scores mentioned in above table will qualify the test and are able to do the visual inspection.
- In-process Quality Assurance person shall observe and verify the whole Qualification activity.
ACCEPTANCE CRITERIA
| Sr. No. | Particulars | Acceptance Criteria |
| 1 | Illumination | NLT 1000 Lux |
| 2 | Black & White back ground | Should be available |
| 3 | Eye test (Test Frequency: Annually) | Qualify the test |
| 4 | Colour blindness (Test Frequency: Annually) | Should be absent |
| 5 | Inspector qualification criteria | If the inspector satisfactory sorts out good and defective tablets on 100% basis then he/she should be considered as qualified as “Visual inspector-Tablets”. |
PROCEDURE:
Visual inspection of bottles is carried out by trained employee/person under visual inspection booth. Bottles are visually inspected for various kinds of possible abnormalities as define above. All visual inspector shall be qualified before induction in visual inspection as per following procedure:
Preparation of bottles for qualification:
Select 20 positive and 80 negative bottles. Negative and positive units were chosen from either from same batch or from different batches which covers all the types of rejection define above. Different qualification bottles shall be prepared.
- A defects album shall be prepared and Training shall be performed to all selected Inspectors. All selected inspectors should be passed in eye test and colour blindness by using the above test samples and defects album.
- Qualification shall be performed by using the above test samples. Keep the entire (positive and negative) bottles in box / crates for individual inspector.
- Gently swirl or invert each individual container, making sure that no air bubbles are introduced.
- Observe for 5 seconds in front of the white panel. Repeat in front of the black panel.
- Keep the rejected bottles in different crates after visual Inspection.
- After completion of visual inspection of bottles, stop the activity and inspect rejected bottles and find out whether all the known rejection recovered during checking or not.
- If all the known rejected bottles, ampoules recovered/qualify the acceptance criteria during visual inspection that means that the visual inspector is fit for visual inspection process.
VISUAL INSPECTION RECORD
Visual Inspector name
| Evaluation Table | |||
| Parameters | Description | Bottles Number | Observation Number |
| True Positive (TP) | Total defective Bottles | 20 | Total Bottles of Visual Inspection kit = 100 |
| True Negative (TN) | Total good Bottles | 80 | |
| False Positive (FP) | Good Bottles counted as defective | ||
| False Negative (FN) | Defective Bottles counted as good | ||
Result: Qualified / Not qualified.
Datasheet #5 Visual inspector’s record
VISUAL CONDITIONS:
- NLT 300 Lux for tablet inspection table.
CLASSIFICATION OF REJECTIONS:
| Type of Rejection | For Oral Solid Dosage Form (Coated Tablet). | For Oral Solid Dosage Form (Uncoated Tablet). |
| Critical | Foreign product, foreign material.
Tablet not in uniform size. Missing debossing. Abnormal discoloration of products. Large dark staining on product. |
Foreign product, foreign material.
Wrong appearance. Tablet not in uniform size / Wrong punch shape. Abnormal / discoloration of products. |
| Major | Blooming, Bridging.
Chipping. Color Variation/ Mottling. Cratering. Flaking. Orange Peel/Roughness. Splitting. Sticking. Twinning. |
Chipping or Minor breaking.
Illegible de-bossing. Illegible embossing. Layer separation. Lamination. Cracking / Broken tablet. Double impression. Soft tablets. Picking. Sticking. Dark spot / Black spot / Color particles. Capping. Binding. |
| Minor | Blushing
Cracking/Splitting. Peel Off. Pitting. Shade variation, Infilling. |
Mottling.
Rough surface. Shade Variation. Shade variation. Dust on Tablet. De-bossing or score is not well defined. |
SELECTION OF VISUAL INSPECTOR-
- All visual inspectors had to gone through the eye medical checkup in order to assess visual acuity.
- Color blindness of each personnel shall be verified.
- Each trainee shall be followed by training programmed for visual inspections.
- All visual inspectors had to gone through the visual inspection kit in order to assess visual acuity.
ACCEPTANCE CRITERIA:
| Sr. No. | Particulars | Acceptance Criteria |
| 1 | Illumination | NLT 300 Lux |
| 2 | Eye test (Test Frequency: annually) | Qualify the test |
| 3 | Colour blindness (Test Frequency: annually) | Should be absent |
| 4 | Inspector qualification criteria | If the inspector satisfactory sorts out good and defective tablets on 100% basis then he/she should be considered as qualified as “Visual inspector-Tablets”. |
PROCEDURE:
Visual inspection of tablets is carried out by trained employee/person on visual inspection table. Tablets are visually inspected for various kinds of possible abnormalities as define above. All visual inspector shall be qualified before induction in visual inspection as per following procedure:
Preparation of tablets for qualification:
Select 20 positive and 80 negative tablets.. Negative and positive units were chosen from either from same batch or from different batches which covers all the types of rejection define above. Different qualification tablets shall be prepared.
- A defects album shall be prepared and Training shall be performed to all selected Inspectors. All selected inspectors should be passed in eye test and colour blindness by using the above test samples and defects album.
- Qualification shall be performed by using the above test samples. Keep the entire (positive and negative) tablets in box / crates for individual inspector.
- Keep the rejected tablets in rejection box after visual Inspection.
- After completion of visual inspection of tablets, stop the activity and inspect rejected tablets and find out whether all the known rejection recovered during checking or not.
- If all the known rejected tablets, tablets recovered/qualify the acceptance criteria during visual inspection that means that the visual inspector is fit for visual inspection process.
Datasheet #6 VISUAL INSPECTORS LIST
| VISUAL INSPECTORS LIST | |||
| Sr. No. | Name of Person | Qualification Status | Remarks |
GxP in Pharmaceuticals: Ensuring Quality, Safety, and Compliance

