Wockhardt Limited- Walk In Interview For Chemist to Asst Manager – Production on 23rd June 2025
Walk-In Interview Details:
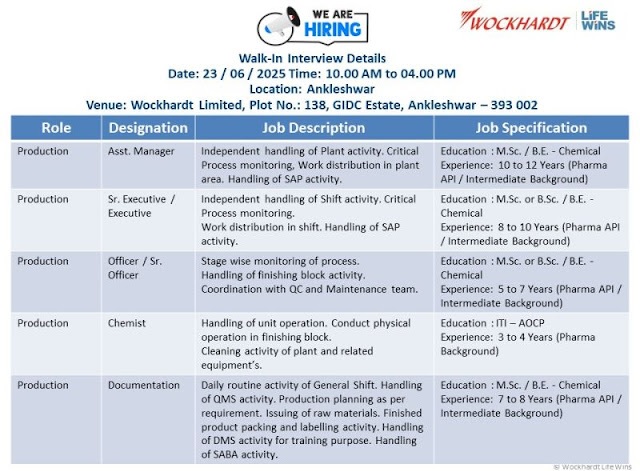
Date: 23rd June 2025
Time: 10:00 AM to 04:00 PM
Location: Ankleshwar
Venue: Wockhardt Limited, Plot No.: 138, GIDC Estate, Ankleshwar – 393 002
1) Production – Asst. Manager Independent handling of plant activity, critical process monitoring, and work distribution in the plant area. Responsible for SAP activity management.
Qualifications: M.Sc. / B.E. – Chemical
Experience: 10 to 12 years in Pharma API / Intermediate background
2) Production – Sr. Executive / Executive Managing shift activities, critical process monitoring, and work distribution. Oversight of SAP-related tasks.
Qualifications: M.Sc. or B.Sc. / B.E. – Chemical
Experience: 8 to 10 years in Pharma API / Intermediate background
3) Production – Officer / Sr. Officer Stage-wise process monitoring, handling finishing block activities, and coordination with QC and maintenance teams.
Qualifications: M.Sc. or B.Sc. / B.E. – Chemical
Experience: 5 to 7 years in Pharma API / Intermediate background
4) Production – Chemist Managing unit operations, conducting physical operations in the finishing block, and ensuring plant and equipment cleanliness.
Qualifications: ITI – AOCP
Experience: 3 to 4 years in Pharma background
5) Production – Documentation General shift routine tasks, QMS handling, production planning, raw material issuance, finished product packaging, labeling, and training document management through DMS. Managing SABA activity
Qualifications: M.Sc. / B.E. – Chemical
Experience: 7 to 8 years in Pharma API / Intermediate background