Virchow Biotech – Interview For Quality Control Dept on 14 & 15th june 2024
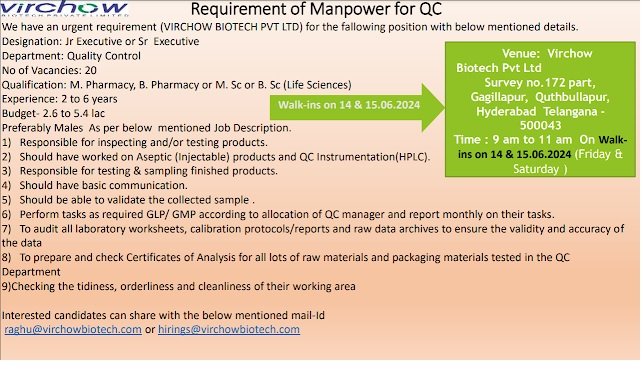
We have an urgent requirement (VIRCHOW BIOTECH PVT LTD) for the fallowing position with below mentioned details.
Designation: Jr Executive or Sr Executive
Department: Quality Control
No of Vacancies: 20
Qualification: M. Pharmacy, B. Pharmacy or M. Sc or B. Sc (Life Sciences)
Experience: 2 to 6 years
Budget- 2.6 to 5.4 lac
Preferably Males As per below mentioned Job Description.
1) Responsible for inspecting and/or testing products.
2) Should have worked on Aseptic (Injectable) products and QC Instrumentation(HPLC).
3) Responsible for testing & sampling finished products.
4) Should have basic communication.
5) Should be able to validate the collected sample .
6) Perform tasks as required GLP/ GMP according to allocation of QC manager and report monthly on their tasks.
7) To audit all laboratory worksheets, calibration protocols/reports and raw data archives to ensure the validity and accuracy of the data
8) To prepare and check Certificates of Analysis for all lots of raw materials and packaging materials tested in the QC Department
9) Checking the tidiness, orderliness and cleanliness of their working area
Venue: Virchow Biotech Pvt Ltd Survey no.172 part, Gagillapur, Quthbullapur, Hyderabad Telangana -500043
Time : 9 am to 11 am On Walkins on 14 & 15.06.2024
(Friday & Saturday )