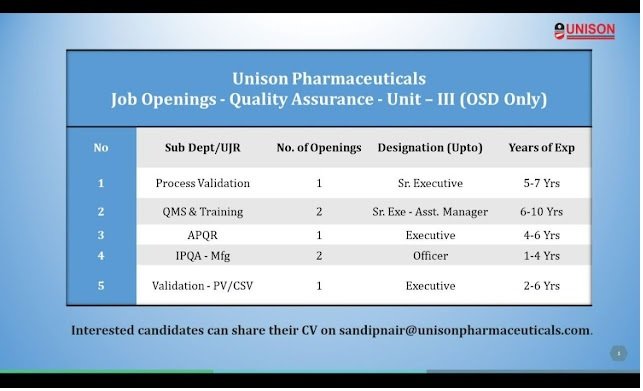
Unison Pharma- Hiring For Quality Assurance Dept
Department : Quality Assurance
1) Sub Dept/UJR : Process Validation
No. of Openings : 1
Designation (Upto) : Sr. Executive
Years of Experience : 5-7 Yrs
2) Sub Dept/UJR : QMS & Training
No. of Openings : 2
Designation (Upto) : Sr. Exe – Asst. Manager
Years of Experience : 6-10 Yrs
3) Sub Dept/UJR : APQR
No. of Openings : 1
Designation (Upto) : Executive
Years of Experience : 4-6 Yrs
4) Sub Dept/UJR : IPQA – Mfg
No. of Openings : 2
Designation (Upto) : Officer
Years of Experience : 1-4 Yrs
5) Sub Dept/UJR : Validation – PV/CSV
No. of Openings: 1
Designation (Upto): Executive
Years of Experience : 2-6 Yrs
Interested candidates can share their CV on sandipnair@unisonpharmaceuticals.com.
Job Category: pharma
Job Type: Full Time
Job Location: india