UNI MEDICOLABS,-interview for Quality Assurance/Production on 24 Feb 2024
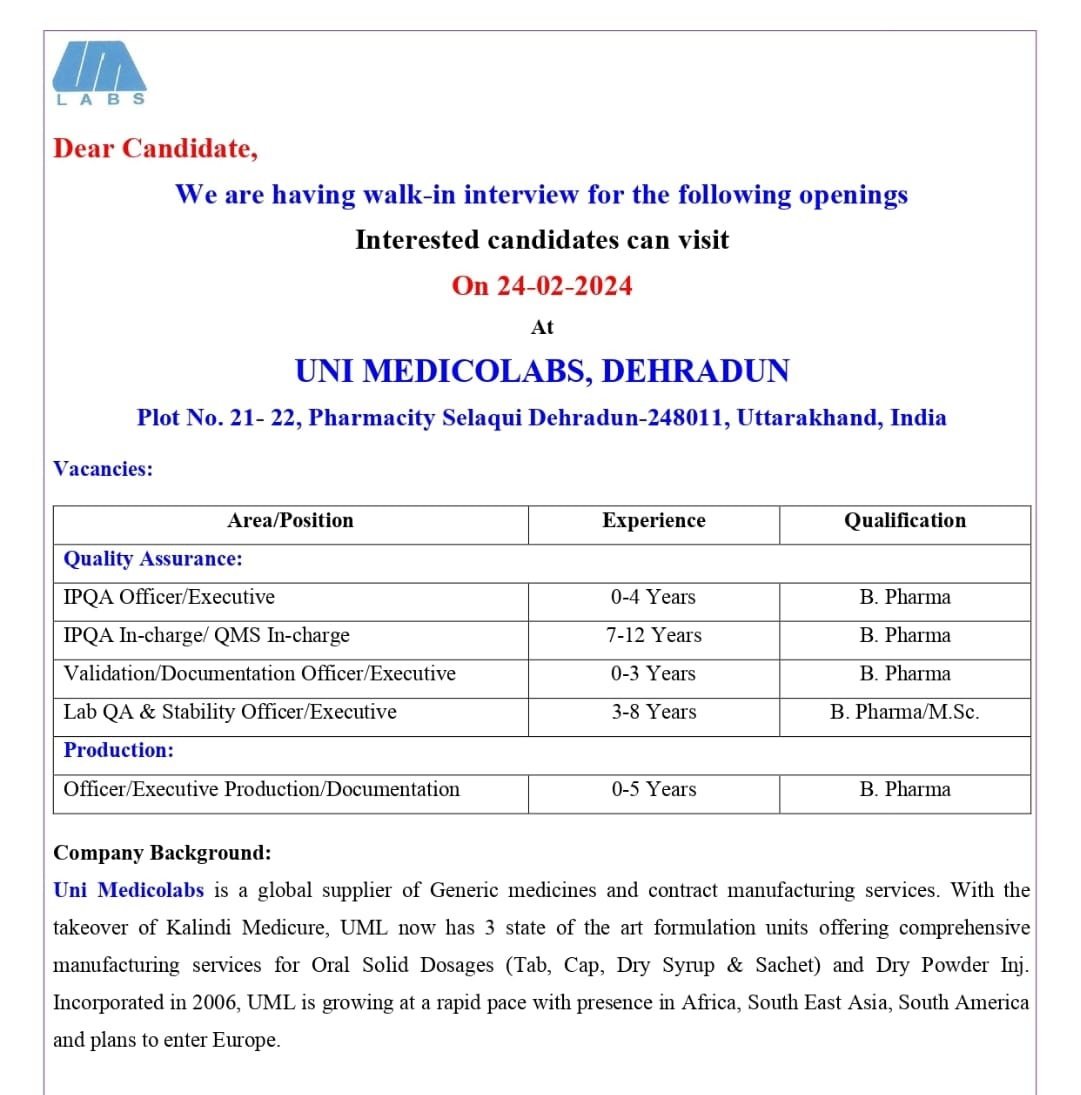
We are having walk-in interview for the following openings
Interested candidates can visit
On 24-02-2024
Venue
UNI MEDICOLABS, DEHRADUN
Plot No. 21-22, Pharmacity Selaqui Dehradun-248011, Uttarakhand, India
Vacancies:
Area/Position
Quality Assurance:
IPQA Officer/Executive-0-4 Years/B. Pharma
IPQA In-charge/ QMS In-charge-7-12 Years/B. Pharma
Validation/Documentation Officer/Executive-0-3 Years/B. Pharma
Lab QA & Stability Officer/Executive-3-8 Years/B. Pharma/M.Sc.
Production:
Officer/Executive Production/Documentation-0-5 Years/B.Pharma
Company Background:Uni Medicolabs is a global supplier of Generic medicines and contract manufacturing services. With the takeover of Kalindi Medicure, UML now has 3 state of the art formulation units offering comprehensive manufacturing services for Oral Solid Dosages (Tab, Cap, Dry Syrup & Sachet) and Dry Powder Inj. Incorporated in 2006, UML is growing at a rapid pace with presence in Africa, South East Asia, South America and plans to enter Europe.

