Torrent Pharma -Interview for M.Pharm, MSc in Regulatory Affairs, ADL, F&D, Pharmacokinetic Unit on 25th February 2024
Torrent Pharma, the flagship Company of Torrent Group is one of the leading pharma companies of the Country. The Company was a pioneer in initiating the concept of niche marketing in India and today is ranked amongst the leaders in therapeutic segment of cardiovascular (CV), central nervous system (CNS), gastro-intestinal (GI) and women healthcare (WHC). The Company also has significant presence in diabetology, pain management, gynaecology, oncology and anti-infective segments.
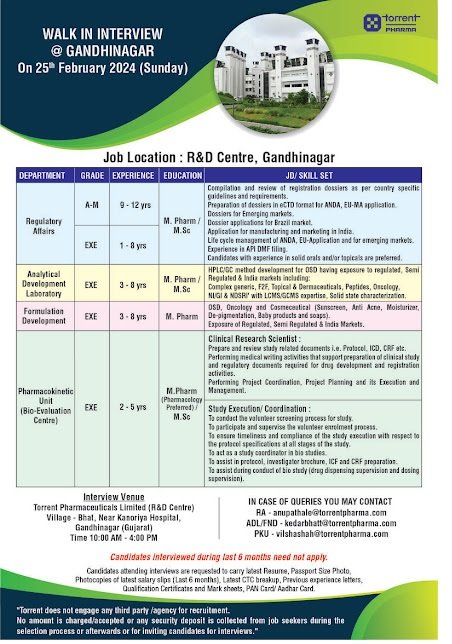
Regulatory Affairs Department
Experience : Area Manager : 9-12 years
Executive : 1-8 years
Education : M.Pharm / M.Sc
Jd/ Skill Set : • Compilation and review of registration dossiers as per country specific guidelines and requirements.
• Preparation of dossiers in eCTD format for ANDA, EU-MA application. Dossiers for Emerging markets.
• Dossier applications for Brazil market.
• Application for manufacturing and marketing in India.
• Life cycle management of ANDA, EU-Application and for emerging markets. Experience in API DMF filing.
• Candidates with experience in solid orals and/or topicals are preferred.
Analytical Development Laboratory
Post : Executive
Experience : 3 – 8 yrs
Education : M.Pharm / M.Sc
Jd/ Skill Set : HPLC/GC method development for OSD having exposure to regulated. Semi Regulated & India markets including: Complex generic, F2F, Topical & Dermaceuticals, Peptides, Oncology, NI/GI & NDSRI’ with LCMS/GCMS expertise. Solid state characterization.
Formulation Development
Post : Executive
Experience : 3 – 8 yrs
Education : M.Pharm
Jd/ Skill Set : OSD, Oncology and Cosmeceutical (Sunscreen, Anti Acne, Moisturizer, De-pigmentation, Baby products and soaps). Exposure of Regulated, Semi Regulated & India Markets.
Pharmacokinetic Unit (Bio-Evaluation Centre)
Post : Executive
Experience : 2 – 5 yrs
Education : M.Pharm (Pharmacology Preferred)/ M.Sc
Jd/ Skill Set : Clinical Research Scientist
Prepare and review study related documents i.e. Protocol, ICD, CRF etc. Performing medical writing activities that support preparation of clinical study and regulatory documents required for drug development and registration activities.
Performing Project Coordination, Project Planning and its Execution and Management.
Study Execution / Coordination
To conduct the volunteer screening process for study.
To participate and supervise the volunteer enrolment process.
To ensure timeliness and compliance of the study execution with respect to the protocol specifications at all stages of the study.
To act as a study coordinator in bio studies.
To assist in protocol, investigator brochure, ICF and CRF preparation.
To assist during the conduct of bio study (drug dispensing supervision and dosing supervision).
Job Location : R&D Centre, Gandhinagar
Walk in Interview
Date : 25th February 2024 (Sunday)
Time : 10:00 AM-4:00 PM
Venue : Torrent Pharmaceuticals Limited (R&D Centre) Village – Bhat, Near Kanoriya Hospital, Gandhinagar (Gujarat)
Candidates interviewed during last 6 months need not apply.
Candidates attending interviews are requested to carry latest Resume, Passport Size Photo, Photocopies of latest salary slips (Last 6 months), Latest CTC breakup, Previous experience letters, Qualification Certificates and Mark sheets, PAN Card/ Aadhar Card.
IN CASE OF QUERIES YOU MAY CONTACT
RA – anupathale@torrentpharma.com
ADL/FND – kedarbhatt@torrentpharma.com
PKU – vilshashah@torrentpharma.com

