Titan Laboratories Pvt Ltd -Interview for Multiple Positions on 21st July 24
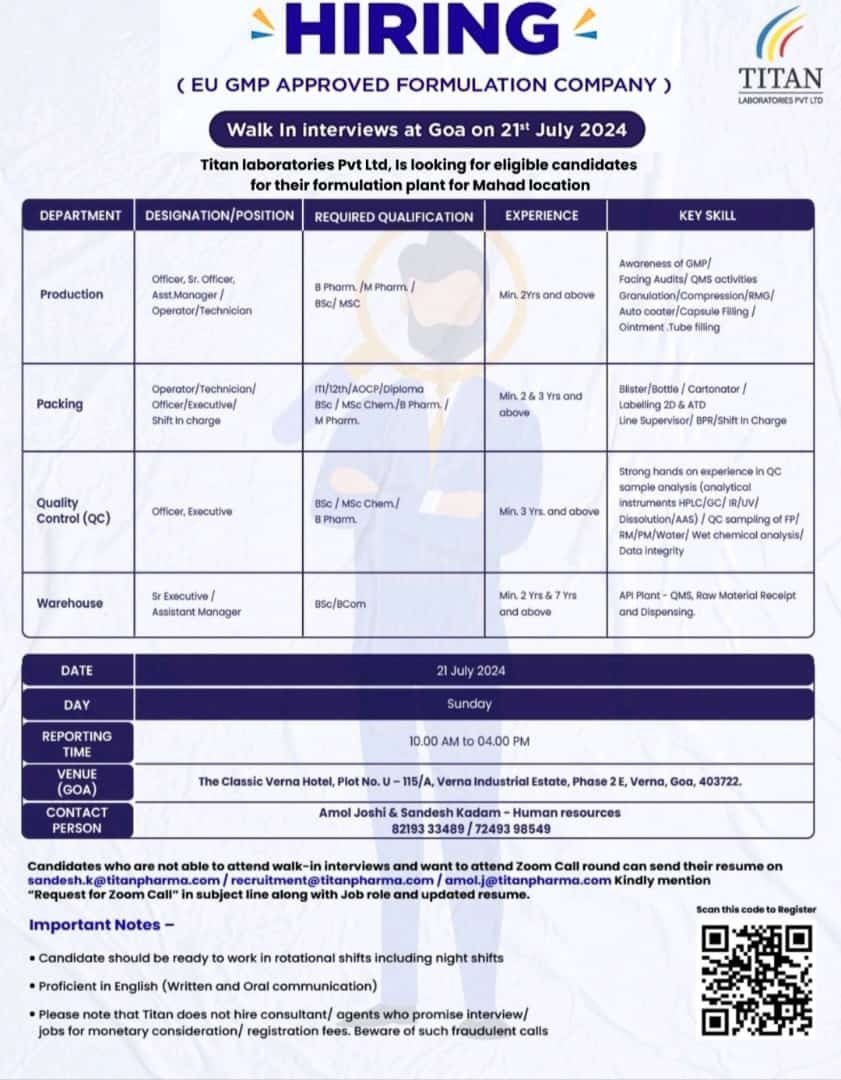
Job Description: Titan Laboratories Pvt. Ltd, Is looking for eligible candidates for their formulation plant for Mahad location.
• Qualification: B.Sc/M.Sc/ B.Pharm/ M.Pharm/B.Com/ITI/12th / IAOCP/
Diploma
• Experience: 02-07 years
• Role: Officer, Sr. Officer, Operator, Technician, Executive, Asst. Manager
• Department: Production/Packing/Quality Control / Warehouse •Work Location: Mahad Raigad, Maharashtra
Walk-In Interview Details:
. Date: 21 July 2024 & Sunday
.Time: 10.00 AM to 04.00 PM
• Venue: The Classic Verna Hotel, Plot No. U-115/A, Verna Industrial Estate, Phase 2 E. Verna, Goa, 403722.
Candidates who are not able to attend walk-in interviews and want to attend Zoom Call round can send their resume on sandesh.k@titanpharma.com,
recruitment@titanpharma.com, or amol.j@titanpharma.com.
Please mention “Request for Zoom Call” in the subject line of your email. Scan the code to Register for the event!
Important Notes:
. Scan this code to Register
. Candidate should be ready to work in rotational shifts including night shifts
• Proficient in English (Written and Oral communication)
Please note that Titan does not hire consultant/ agents who promise interview/jobs for monetary consideration/registration fees. Beware of such fraudulent calls.
