SYMED LABS LIMITED –Interview for FRESHERS – Apprentices on 29th & 30th Mar’ 2024
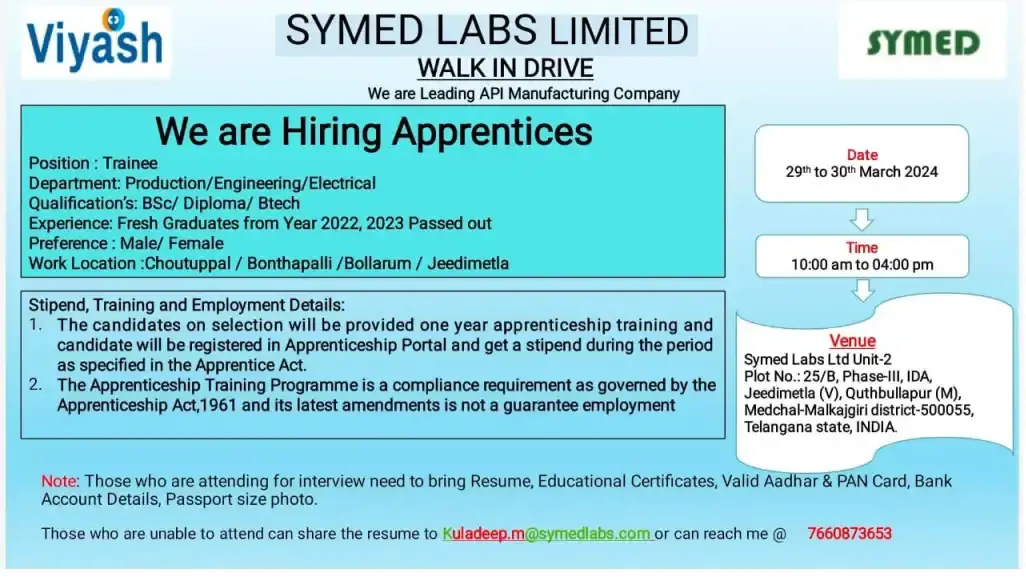
Position Available: Trainee
Departments:Production/Engineering/Electrical
Qualifications:BSc/ Diploma/ B.Tech
Experience:Fresh graduates from the years 2022 and 2023
Preference: Male/Female candidates
Work Locations:Choutuppal/Bonthapalli/Bollarum/Jeedimetla
Walk-In Interview Details: Date: 29th to 30th March 2024
Time: 10:00 am to 04:00 pm
Venue: Symed Labs Ltd Unit-2 Plot No.: 25/B, Phase-III, IDA, Jeedimetla (V), Quthbullapur (M), Medchal-Malkajgiri district-500055, Telangana state, INDIA.
Stipend, Training, and Employment Details: Selected candidates will undergo a one-year apprenticeship training program as per the guidelines of the Apprenticeship Act, 1961. The candidates will receive a stipend during the training period. Please note that completion of the apprenticeship training program does not guarantee employment.
Note to Candidates:
- Bring resume, educational certificates, valid Aadhar & PAN card, bank account details, and passport-size photo to the interview.
- If unable to attend the walk-in interview, candidates can share their resumes via email to Kuladeep.m@symedlabs.com or contact us at 7660873653.

