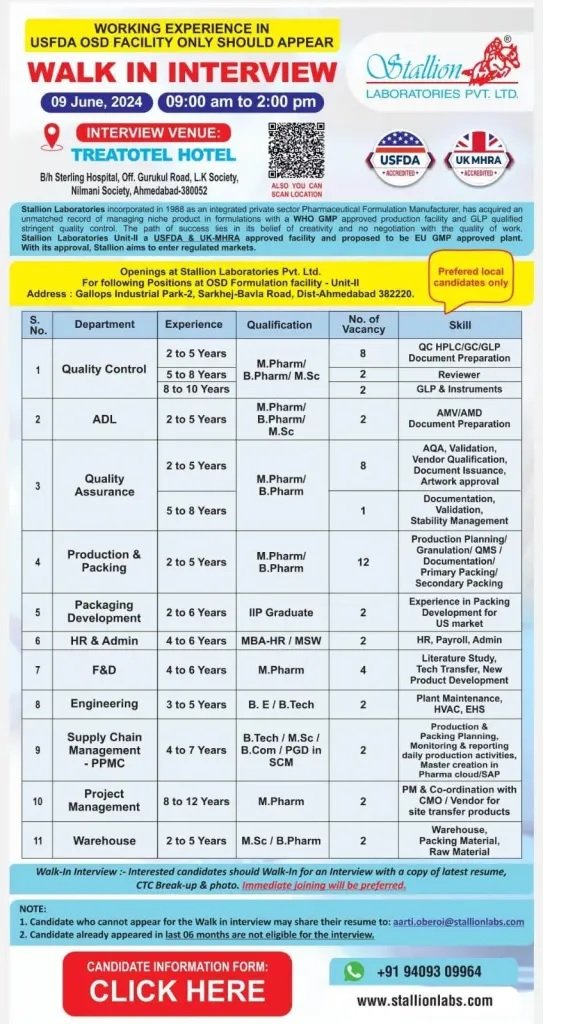
Stallion Laboratories – interview for multiple openings on 09th June, 2024 @ Ahmedabad
Stallion Laboratories walk in interview for multiple openings on 09th June, 2024 @ Ahmedabad
Stallion Laboratories incorporated in as an integrated private sector Pharmaceutical Formulation Manufacturer, has acquired an ursmutihod record of
Openings at Stallion Laboratories Pvt. Ltd.
For following Positions at OSD Formulation facility – Unit-ll Address: Gallops Industrial Park-2, Sarkhej-Bavia Road, Dist-Ahmedabad 382220.
Department: Quality Control, Quality Assurance, ADL, Production & Packing, F&D, Engineering, Packaging Development, HR & Admin, Warehouse, Project Management, Supply Chain Management -PPMC
Experience: 2 to 12 Years
Qualifications: B.Tech/ M.Sc/ B.Com/ PGD in SCM/ MBA-HR/ MSW/ M.Pharm/ B.Pharm/ M.Sc
Date: 09 June, 2024, 09:00 am to 2:00 pm
INTERVIEW VENUE:TREATOTEL HOTEL B/h Sterling Hospital, Off. Gurukul Road, LK Society, Nilmani Society, Ahmedabad- 380052
Walk-in Interview interested candidates should Walk-in for an interview with a copy of latest resume,
CTC Break-up & phate, immediate joining will be preferred.
NOTE 1. Candidate who cannot appear for the Walk in interview may share their resume to: aarti.oberoi@stallionlabs.com
2. Candidate already appeared in last 06 months are not eligible for the interview.