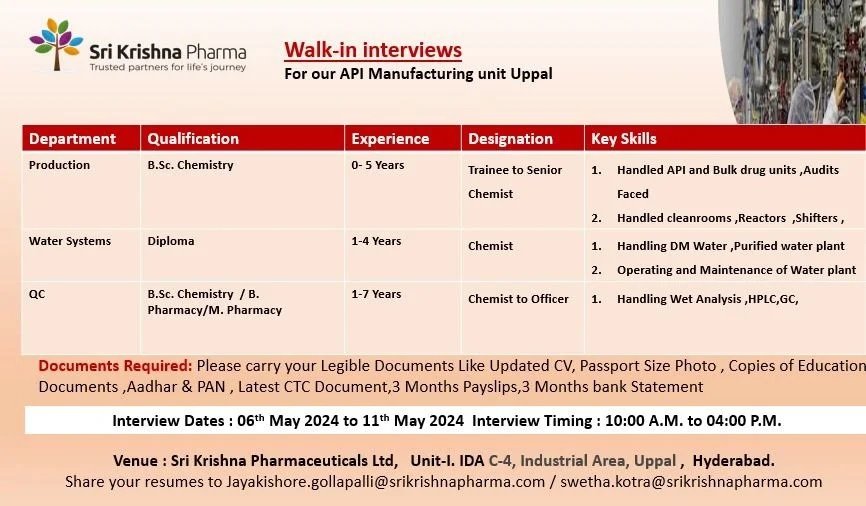
Sri Krishna Pharmaceuticals -interview for Freshers and Experience on 6th to11th May 2024
We are conducting walk-in interview for Freshers and Experienced candidates on 6th May to 11th May 2024. Interested and Eligible candidates can attend interview at Hyderabad.
Department: Production
Role: Trainee to Sr. Chemist
Experience: 0 – 5 years
Qualification: B.Sc. Chemistry
Key Skills:
- Handled API and Bulk drug units, Audits faced.
- Handled cleanrooms, Reactors, Shifters
Department: Water System
Role: Chemist
Experience: 1 – 4 years
Qualification: Diploma
Key Skills:
- Handling DM water, Purified water plant.
- Operating and Maintenance of water plant.\
Department: Quality Control
Role: Chemist to Officer
Experience: 1 – 7 years
Qualification: B.Sc. Chemistry/ B Pharmacy/ M Pharmacy
Key Skills:
- Handling Wet analysis, HPLC, GC
Walk-in interview details
Interview Dates: 06th May 2024 to 11th May 2024
Interview Timing: 10:00 A.M. to 04:00 P.M.
Venue: Sri Krishna Pharmaceuticals Ltd, Unit-I. IDA C-4, Industrial Area, Uppal , Hyderabad.
Documents Required: Please carry your Legible Documents Like Updated CV, Passport Size Photo , Copies of Education, Documents, Aadhar & PAN, Latest CTC Document, 3 Months Payslips, 3 Months bank Statement
Share your resumes to [email protected] / [email protected]