Satyadivis Pharmaceuticals-Interviews for Freshers in Production / Maintenance on 1st to 5th July, 2025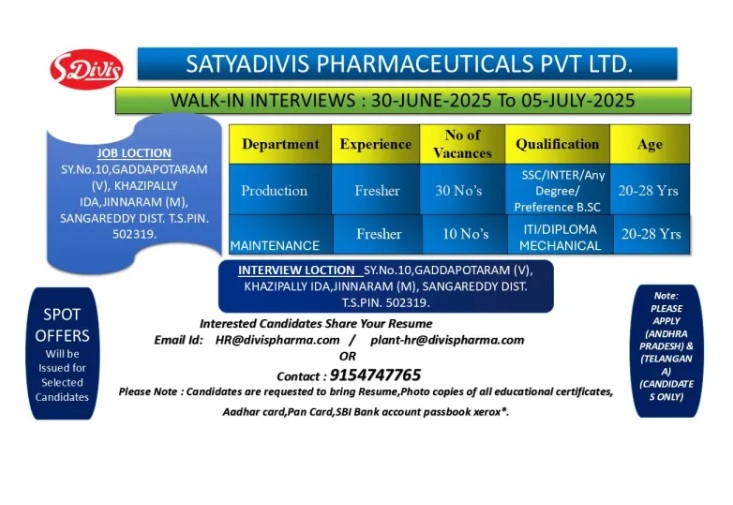
WALK-IN-INTERVIEW @ Satyadivis Pharmaceuticals Pvt. Ltd Interviews will be held on 1st to 5th July, 2025 for Freshers in Production / Maintenance Departments at Hyderabad.
Job description:
Department: Production / Maintenance
. No of Positions: 40
. Qualification: B.Sc, SSC, Inter, ITI, Diploma, Any Degree
. Experiences: Freshers
⚫ Job Location: Kazipally (V), Jinnaram (M), Sangareddy (Dist), Telangana. . Male Candidates Only & AP & TS People only
Walk-In Interview Details:
. Date: 1st to 5th July, 2025
.Time: 09:30am to 05:30pm
. Venue: Satyadivis Pharmaceuticals Pvt. Ltd. Sy.No.10, Gaddapotaram (V), Khazipally IDA, Jinnaram (M), Sangareddy Dist. T.S. PIN. 502319.
Map Link: Satyadivis Pharmaceuticals Pvt. Ltd.
. Interested Candidates Share Your Resume Email Id: [email protected] / plant- [email protected] Contact: 9154747765
