Mendas Pharma – Interview For BE/ Diploma Chemical/ MSc/ BSc/ B Pharm/ M Pharm/ AOCP on 18th February, 2024
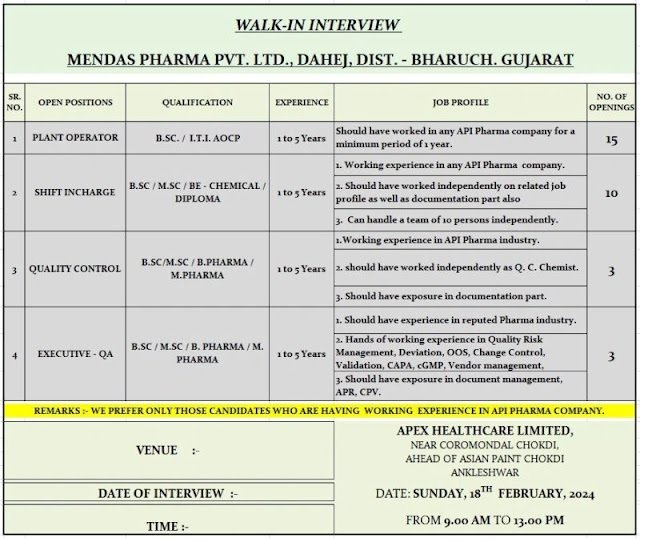
WALK-IN-INTERVIEW
MENDAS PHARMA PVT. LTD., DAHEJ, DIST. – BHARUCH. GUJARAT
OPEN POSITIONS-PLANT OPERATOR/SHIFT INCHARGE/QUALITY CONTROL/EXECUTIVE-QA
EXPERIENCE-1 to 5 Years
QUALIFICATION–B.SC. / I.T.I. AOCP
B.SC/M.SC/BE-CHEMICAL/ DIPLOMA/B.SC/M.SC/B.PHARMA/ M.PHARMA
JOB PROFILE
Should have worked in any API Pharma company for a minimum period of 1 year.
1. Working experience in any API Pharma company. 2. Should have worked independently on related job profile as well as documentation part also
3. Can handle a team of 10 persons independently. 1.Working experience in API Pharma industry.
2. should have worked independently as Q. C. Chemist. 3. Should have exposure in documentation part.
1. Should have experience in reputed Pharma industry.
2. Hands of working experience in Quality Risk
Management, Deviation, OOS, Change Control, Validation, CAPA, CGMP, Vendor management, 3. Should have exposure in document management, APR, CPV.
REMARKS: WE PREFER ONLY THOSE CANDIDATES WHO ARE HAVING WORKING EXPERIENCE IN API PHARMA COMPANY.
VENUE :-
APEX HEALTHCARE LIMITED,
NEAR COROMONDAL CHOKDI, AHEAD OF ASIAN PAINT CHOKDI ANKLESHWAR
DATE: SUNDAY, 18″ FEBRUARY, 2024
FROM 9.00 AM TO 13.00 PM
3

