LUPIN LTD-Interview for Production, Quality Control Quality Control (Formulation), Engineering Instrumentation (OSD/API) for Mandideep (Bhopal) Plant@ VIZAG on 21st APRIL 2024
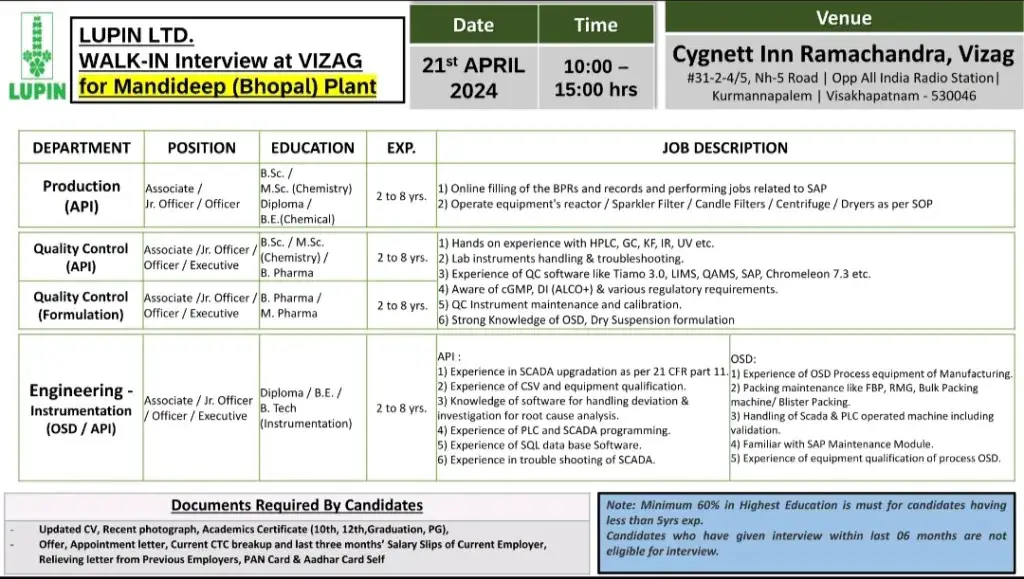
LUPIN LTD. WALK-IN Interview at VIZAG for Mandideep (Bhopal) Plant on 21st April 2024
WE ARE HIRING FOR LUPIN LTD – MANDIDEEP (BHOPAL)
Walk-in directly at the venue, if you meet the eligibility criteria mentioned below.
Department: Production (API), Quality Control (API), Quality Control (Formulation), Engineering Instrumentation (OSD/API)
Position: Associate /Jr. Officer/ Officer/Executive
Education: B. Pharma/ M. Pharma/ B. Sc/ M.Sc/ BE/ B.Tech
Experience: 2 to 8 yrs
Date: 21st APRIL 2024
Time: 10:00 15:00 hrs
Venue:Cygnett Inn Ramachandra, Vizag #31-2-4/5, Nh-5 Road | Opp All India Radio Station|
Kurmannapalem | Visakhapatnam – 530046
Documents Required By Candidates
Updated CV, Recent photograph, Academics Certificate (10th, 12th, Graduation, PG),
Offer, Appointment letter, Current CTC breakup and last three months’ Salary Slips of Current Employer, Relieving letter from Previous Employers, PAN Card & Aadhar Card Self
Note: Minimum 60% in Highest Education is must for candidates having
less than 5yrs exp.
Candidates who have given interview within last 06 months are not eligible for interview.