Ipca Laboratories-Walk-Ins for Trainee/Apprentice Position on 19th Oct’2024
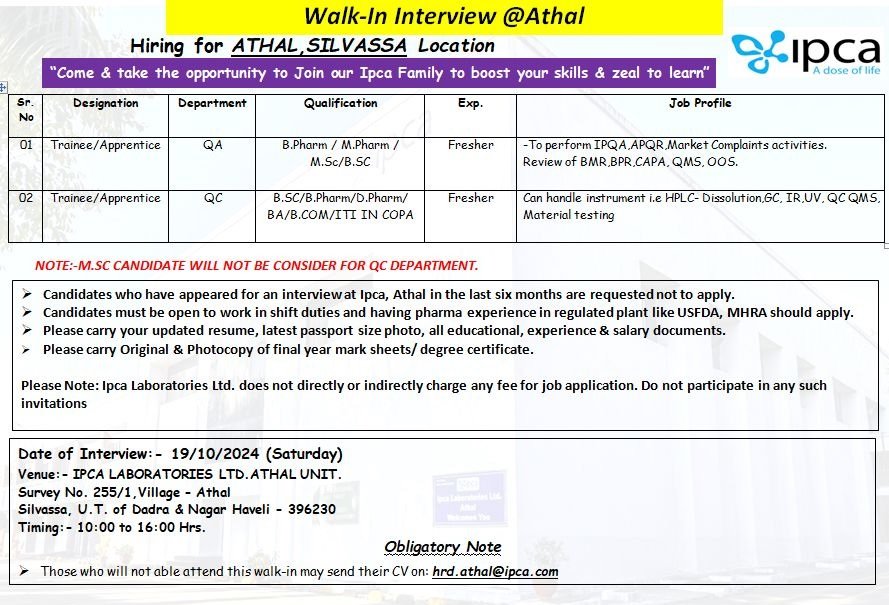
Take the opportunity to join the Ipca family to boost your skills and zeal to learn. We are looking for freshers to join us for the following positions:
1. Trainee/Apprentice – QA Department
- Qualification: B.Pharm / M.Pharm / M.Sc / B.Sc
- Experience: Fresher
- Job Profile: Perform IPQA, APQR, Market Complaints activities. Review of BMR, BPR, CAPA, QMS, OOS.
2. Trainee/Apprentice – QC Department
- Qualification: B.Sc / B.Pharm / D.Pharm / BA / B.Com / ITI in COPA
- Experience: Fresher
- Job Profile: Handle instruments like HPLC, Dissolution, GC, IR, UV, QC QMS, material testing.
Important Notes:
- M.Sc candidates will not be considered for the QC department.
- Candidates who have appeared for an interview at Ipca Athal in the last 6 months should not apply.
- Open to work in shifts with pharma experience in regulated plants like USFDA, MHRA.
- Bring updated resume, passport-sized photo, and all relevant educational and experience documents.
- Original and photocopy of final year mark sheets/degree certificates are required.
- Date: 19/10/2024 (Saturday)
- Venue: Ipca Laboratories Ltd., Athal Unit, Survey No. 255/1, Village – Athal, Silvassa, U.T. of Dadra & Nagar Haveli – 396230
- Time: 10:00 AM to 4:00 PM
Candidates unable to attend the walk-in may send their CV to: hrd.athal@ipca.com
Job Category: pharma
Job Type: Full Time
Job Location: Dadra & Nagar Haveli