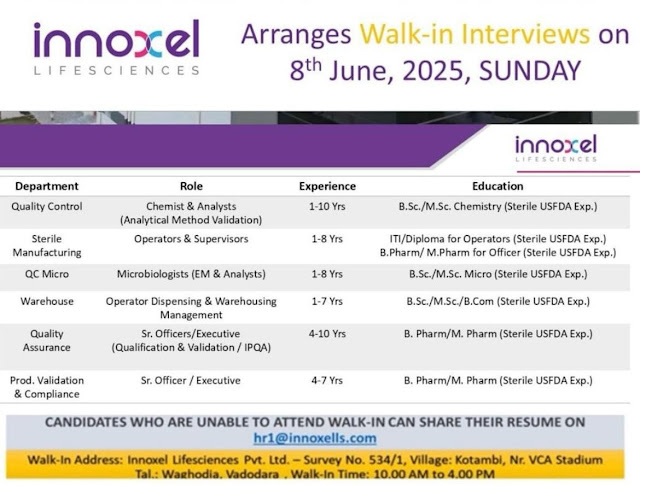
Innoxel Lifesciences- Walk-in Interview for Production, QA, QC, QC-Micro, Warehouse & Manufacturing on 08 June 2025 @Vadodara
Qualifications: BSc/MSc in Chemistry
Experience: 1-10 years (Sterile USFDA experience)
Roles: Chemist & Analyst
2. Sterile Manufacturing:
Qualifications: ITI/Diploma for Operators; BPharm/MPharm for Officers
Experience: 1-8 years (Sterile USFDA experience)
Roles: Operators & Supervisors
3. Quality Control Microbiology (QC Micro):
Qualifications: BSc/MSc in Microbiology
Experience: 1-8 years (Sterile USFDA experience)
Roles: Microbiologists (EM & Analysts)
4. Warehouse Operations:
Qualifications: BSc/MSc/BCom
Experience: 1-7 years (Sterile USFDA experience)
Roles: Operators for Dispensing & Warehousing Management
5. Quality Assurance (QA):
Qualifications: BPharm/MPharm
Experience: 4-10 years (Sterile USFDA experience)
Roles: Sr. Officers & Executives (Qualification & Validation/IPQA)
6. Production Validation & Compliance:
Qualifications: BPharm/MPharm
Experience: 4-7 years (Sterile USFDA experience)
Roles: Sr. Officer/Executive
Date: June 8, 2025 (Sunday)
Time: 10:00 AM to 4:00 PM
Location: Innoxel Lifesciences Pvt. Ltd., Survey No. 534/1, Kotambi, Nr. VCA Stadium, Waghodia, Vadodara, Gujarat