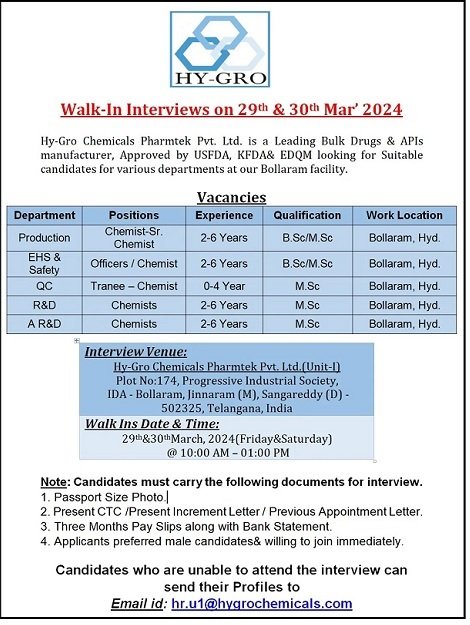
Hy-Gro Chemicals Pharmtek Pvt. Ltd- Interviews for Production/ EHS & Safety/ AR&D/ R&D/ QC On 29th & 30th March 2024
Hy-Gro Chemical Pharmtek Pvt. Ltd is a leading Bulk Drugs & APIs Manufacturer, Approved by USFDA & KFDA, looking for suitable candidates for Quality Control/ Production Department at our Vizag facility.
Walk-In Interviews for freshers & experienced in Production/ EHS & Safety/ AR&D/ R&D/ QC On 29th & 30th March 2024 @ Hy-Gro Chemicals Pharmtek Pvt. Ltd.
Department: Production/ EHS & Safety/ AR&D/ R&D/ QC
Position: Chemist- Sr. Chemist/ Officer/ Trainee
Experience: 0 to 06 years
Qualification: M.Sc/ B.Sc
Work Location: Bollaram, Hyd
Date: 29th & 30th March 2024
Time: 10:00 AM to 01:00 PM
Venue: Hy-Gro Chemicals Pharmtek Pvt. Ltd, (Unit-I), Plot No. 174, Progressive Industrial Society, IDA-Bollaram, Jinnnaram (M), Sangareddy (D)- 502325, Telangana, India
Note: Candidates must carry the following documents to eligible for Interview.
Applicants preferred Male Candidates willing to Join Immediately.
Passport Size Photo.
Present CTC/ Present Increment Letter/ Previous Appointment Letter.
Three Months Pay Slips along with Bank Statement.
- Candidates who are unable to attend the interview can send their Profiles to : hr.u1@hygrochemicals.com

