Hazelo Labs- walk-in drive for Production, QA, QC, R&D, SRS / EHS / ETP / MEE / Stores / Warehouse on 9th & 10th May 2024
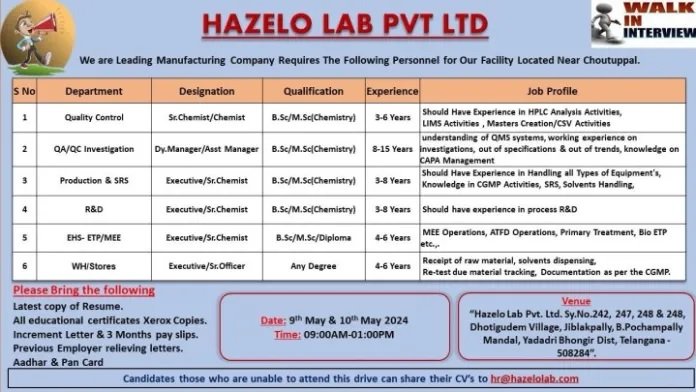
Department : Production / Quality Assurance / Quality Control / R&D / SRS / EHS / ETP / MEE / Stores / Warehouse
Experience : 03-15 Years
Qualification: B.Sc / M.Sc / Diploma / Any Graduates
Walk-In Interview Details:
Date of interview: 9th & 10th May 2023
Time: 09:00 AM-1:00 PM
Venue: HAZELO LAB PRIVATE LIMITED, H.No:-. 4-166, Survey NO:242, 247, 248 & 249, Dothigudam Village, B. Pochampally Mandal, Yadadri Bhongiri District – 508284, Telangana
Documents to be Carried:
- Updated Resume,
- Xerox copies of all Educational Certificates,
- Previous company Appointment/Latest Increment letter,
- last 3 months pay slips,
- last 3 months Bank statement.
Note: Those who are unable to attend can share their resume to [email protected].

