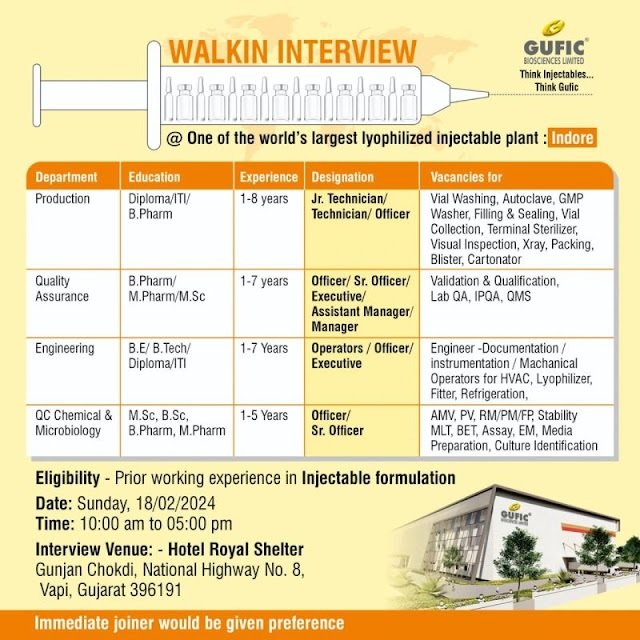
Gufic Biosciences-Interview For Production/ QA/ Engineering/ QC Chemical & Microbiology on 18 Feb 2024
One of the world’s largest lyophilized injectable plant : Indore
1) Department: Production
Education: Diploma/ ITI/ B.Pharm
Experience: 1-8 years
Designation: Jr. Technician/ Technician/ Officer
Vacancies for: Vial Washing, Autoclave, GMP Washer, Filling & Sealing, Vial Collection, Terminal Sterilizer, Visual Inspection, Xray, Packing, Blister, Cartonator
2) Department: Quality Assurance
Education: B.Pharm/ M.Pharm/ M.Sc
Experience: 1-7 years
Designation: Officer/ Sr. Officer/ Executive/ Assistant Manager/ Manager
Vacancies for: Validation & Qualification, Lab QA, IPQA, QMS
3) Department: Engineering
Education: B.E/ B.Tech/ Diploma/ ITI
Experience: 1-7 years
Designation: Operators/ Officer/ Executive
Vacancies for: Engineer- Documentation/ instrumentation / Machanical Operators for HVAC, Lyophilizer, Fitter, Refrigeration
4) Department: QC Chemical & Microbiology
Education: M.Sc, B.Sc, B.Pharm, M.Pharm
Experience: 1-5 Years
Designation: Officer/ Sr. Officer
Vacancies for: AMV, PV, RM/PM/FP, Stability MLT, BET, Assay, EM, Media Preparation, Culture Identification
Eligibility – Prior working experience in Injectable formulation
Date: Sunday, 18/02/2024
Time: 10:00 am to 05:00 pm
Venue: – Hotel Royal Shelter Gunjan Chokdi, National Highway No. 8, Vapi, Gujarat 396191