Gufic Bioscience- Interview Production/ QA/ QC/ Engineering Dept on 9th June 2024
Gufic Group have been in the Pharmaceutical industry since 1970 and are known and respected for Innovative and high Quality pharmaceutical and Herbal Products along with a wide range of APIs Gufic Group are one of the largest manufacturers of Lyophilized injections in India and have a fully automated lyophilization plant Our lyophillized product portfolio includes Antibiotic, Antifungal, Cardiac, Infertility, Antiviral and proton-pump inhibitor segments.
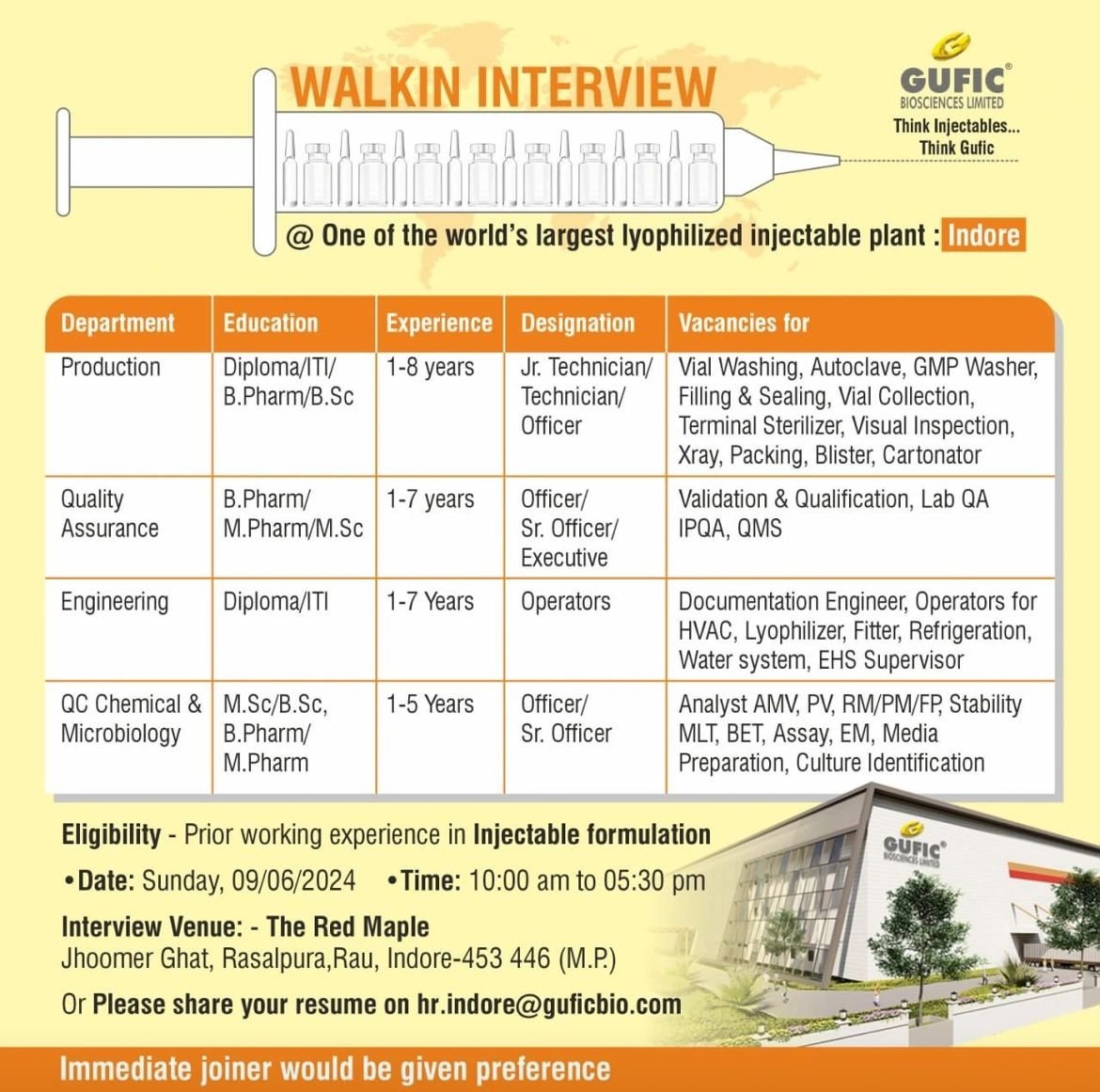
Department: Production
Education: Diploma/ ITI/ B.Pharm/B.Sc
Experience: 1-8 years
Post: Jr. Technician/ Technician/ Officer
vacancies for: Vial Washing, Autoclave, GMP Washer, Filling & Sealing, Vial Collection, Terminal Sterilizer, Visual Inspection, Xray, Packing, Blister, Cartonator
Department: Quality Assurance
Education: B.Pharm/ M.Pharm/M.Sc
Experience: 1-7 years
Post: Officer/ Sr. Officer/ Executive
vacancies for: Validation & Qualification, Lab QA IPQA, QMS
Department: Engineering
Education: Diploma/ITI
Experience: 1-7 Years
Post: Operators
vacancies for: Documentation Engineer, Operators for HVAC, Lyophilizer, Fitter, Refrigeration, Water system, EHS Supervisor
Department: QC Chemical & Microbiology
Education: M.Sc/B.Sc, B.Pharm/ M.Pharm
Experience: 1-5 Years
Post: Officer/ Sr. Officer
vacancies for: Analyst AMV, PV, RM/PM/FR Stability MLT, BET, Assay, EM, Media Preparation, Culture Identification
Eligibility – Prior working experience in Injectable formulation
Date:Sunday, 09/06/2024
Time: 10:00 am to 05:30
Interview Venue: – The Red Maple
Jhoomer Ghat, Rasalpura,Rau, Indore-453 446 (M.P)
Or Please share your resume on hr.indore@guficbio.com
Job Location: Indore