Graviti Pharma-interview for Production, QA, QC, Regulatory Affairs on 14th Apr 2024
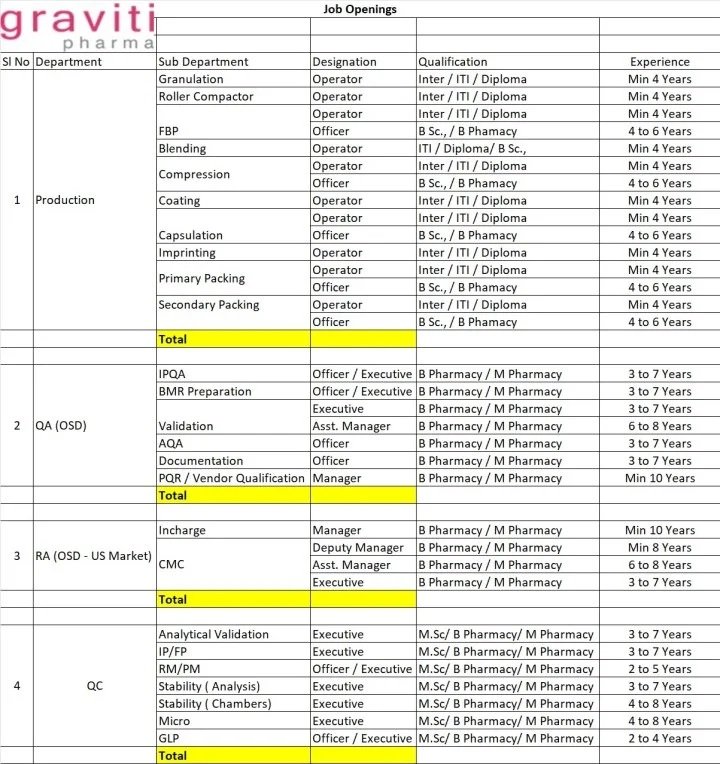
Department: Production / Quality Assurance/ Quality Control/ Regulatory Affairs
Designation: Officer, Operator, Executive, Asst. Manager, Manager
Experience: 2-10 yrs
Qualification: ITI / Diploma/ B.Sc / M.Sc / B.Pharm / M.Pharm
Job Location: Hyderabad
Contact Us:
Free placement (Only OSD Background)
7799807011 / 9849854768
Email: rsconsultants2005@gmail.com
Date of interview: 14-April 2024
Time: 10.00 Am to 3.00 pm
Venue: New Himalaya BanQuet Hall & Restaurant, Near Govt Degree college, Kalwakurthy Road, Jedcherla, Mahbhubnagar, Telangana 509301.
What to bring to the interview:
- Academic documents,
- Pay slips,
- Appointment letter
- ID proof along with photo.

