Felix Generics Pvt Ltd-interview on 26th March 2024
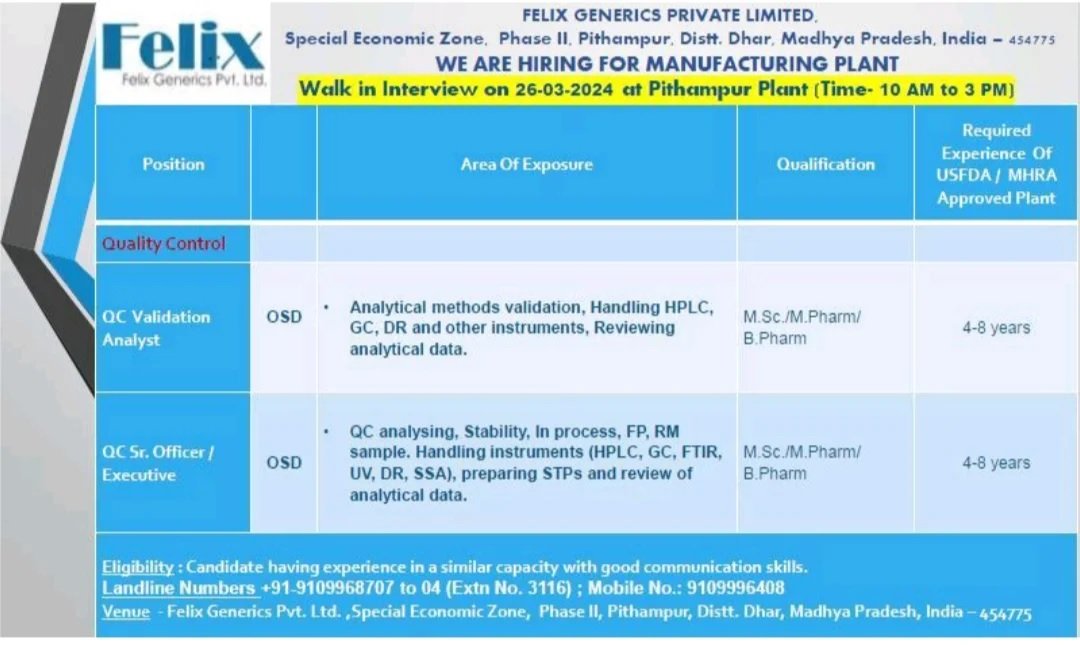
Felix Generics Pvt. Ltd. is hiring for QC Validation Analyst and Sr. Officer/Executive positions. Join us for a walk-in interview on 26-03-2024 at Pithampur Plant. Exciting career opportunities await!
Walk in Interview on 26th March 2024 at Pithampur Plant
Validation Analyst – Quality Control OSD
Area Of Exposure
- Analytical methods validation, Handling HPLC, GC, DR and other instruments, Reviewing analytical data.
Qualification: M.Sc./M.Pharm/ B.Pharm
Experience: 4-8 years of Experience Of USFDA / MHRA Approved Plant
Sr. Officer/ Executive – Quality Control – OSD
Area Of Exposure: QC analysing, Stability, In process, FP, RM sample. Handling instruments (HPLC, GC, FTIR, UV, DR, SSA), preparing STPs and review of analytical data.
Qualification: M.Sc./M.Pharm/ B.Pharm
Experience: 4 – 8 years of Experience of USFDA / MHRA Approved Plant
Eligibility: Candidate having experience in a similar capacity with good communication skills
. Landline Numbers +91-9109968707 to 04 (Extn No. 3116); Mobile No.: 9109996408
Date of interview: 26th March 2024
Time: 10 am to 3 pm
Venue: Felix Generics Pvt. Ltd., Special Economic Zone, Phase II, Pithampur, Distt. Dhar, Madhya Pradesh, India – 454775

