FDC Limited- interviews for Production, Quality Control, Quality Assurance Departments on 10th & 13th April’ 2024
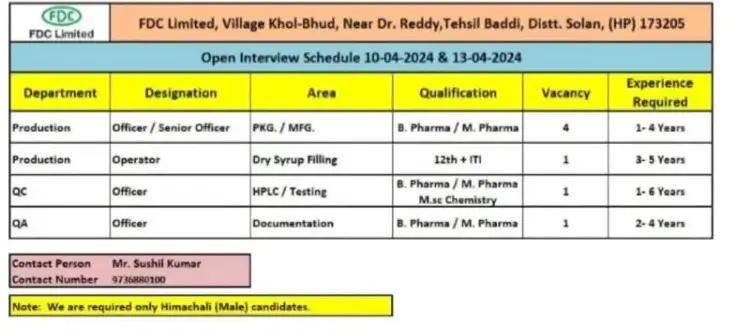
FDC Limited, We are conducting walk-in interviews for Production, Quality Control, Quality Assurance Departments on 10th & 13th April’ 2024 at Khol-Bhud, Tehsil Baddi, Distt. Solan (HP) 173205.
Job Description:
Department: Production, QC, QA
Designation: Operator, Officer/Sr. Officer
• Area: Packing, Manufacturing, Compression, HPLC/TESTING / Reviewer/
Documentation
•No of Positions: 07
Qualification: B.Pharma / M.Pharm, 12th, ITI, M.Sc (Chem.)
Experience: 1-6 Years
Job Location: Baddi, H.P
Interview Schedule Details:
. Date: 10th & 13th April’ 2024
.Time: 10:00 AM to 01:00 PM
. Venue: FDC Limited, Khol-Bhud, Tehsil Baddi, Distt. Solan (HP) 173205
. Contact Person: Mr. Sushil Kumar, Sr. Officer -HR
. Contact Number: 9736880100
Note: We are required only Himachali (Male) Candidates.

