Bacterial Endotoxin Test GTP
Bacterial Endotoxin Test GTP
Bacterial Endotoxin Test GTP
Risk assessment for handling of corticosteroids
RISK ASSESSMENT FMEA/FMECA FOR MEDIA FILL ACTIVITY
RISK ASSESSMENT PROTOCOL FOR RESPULE LINE A risk assessment is a method to assess and characterize the critical parameters in the functionality of a System or Process. Therefore, risk Assessment is a key element in the process and validation approach. Added in product manufacturing process shall be evaluated to identify critical risk at various stages of…
RISK ASSESSMENT PROTOCAL FOR STERILE FORMULATION FACILITY A risk assessment is a method to assess and characterise the critical parameters in the functionality of a System or process. Therefore, risk Assessment is a key element in the process and validation approach. In the Area modification, risk analyses are performed as basic GMP/EHS-Risk Assessment, which shall help…
Read More “RISK ASSESSMENT PROTOCAL FOR STERILE FORMULATION FACILITY” »

SELF-INSPECTION PROGRAMME To describe the procedure for scheduling, conducting a self-inspection activity, and implementing corrective/preventive measures and effective follow-up actions towards the non-compliances observed. DATA INTEGRITY AND DATA RELIABILITY

HANDLING OF EXTERNAL AUDIT OBSERVATIONS AND ITS COMPLIANCE EXTERNAL AUDIT OBSERVATIONS -Compliance shall be filled in proper QMS documents such as CAPA format, Investigation form, deviation, Change control and summary of CAPA shall mentioned in Audit Compliance Report. DATA INTEGRITY AND DATA RELIABILITY
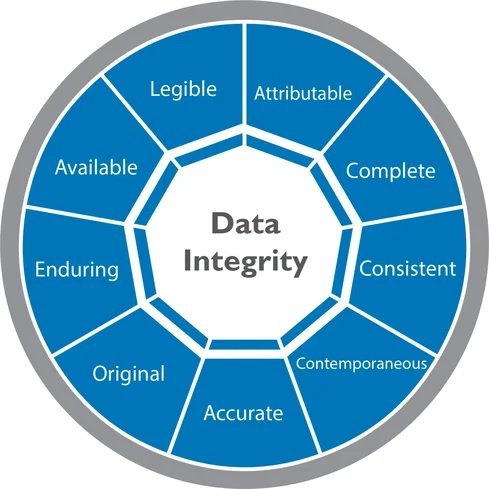
DATA INTEGRITY AND DATA RELIABILITY Data Integrity: The extent to which all data are complete, consistent, and accurate throughout the data lifecycle. Data integrity arrangements must ensure that the accuracy, completeness, content, and meaning of data is retained throughout the data lifecycle. DOCUMENT AND DATA CONTROL PROCEDURE
SOP OF DATA BACKUP AND RESTORATION IN PHARMA OBJECTIVE: To lay down the procedure of data backup & restoration of the Lab Instrument Computer Systems Data Backup Server. SCOPE: The scope of this document is to provide the procedure and alternative procedure for data backup and retrieval from Storage Server Backup Server for QC, Stability, and Micro…
SOP FOR USER ID PASSWORD POLICY IN PHARMA OBJECTIVE: This document provides procedure for issuing user ID’s and Passwords to access Computerized System and procedure to be followed to create, maintain and obsolete user account. SCOPE: This SOP-IT is applicable to all computerized Systems covered under GxP & non GxP. RESPONSIBILITY: T. Administrator: Responsible for implementation of…
SOP ON USER DOMAIN ID IN PHARMA OBJECTIVE: The objective of this policy is to lay down the procedure for issuing domain, internet account ID and password to authorized persons without affecting confidentiality and integrity of organization information residing on Computerized Systems over network. SCOPE: Accounts issued to all employees and contract employees are covered…
SOP for Automatic Ink Jet Sticker Label Overprinting Machine in Pharma Objective: To lay down a procedure for the Operation, Cleaning, and Overprinting of Automatic Ink Jet Sticker Label Overprinting Machine. Scope: This Standard Operating Procedure is applicable for the Operation, Cleaning, and Overprinting Record of the Automatic Ink Jet Sticker Label Overprinting Machine at…
Read More “SOP for Automatic Ink Jet Sticker Label Overprinting Machine in Pharma ” »
Operation, Cleaning, and Overprinting of Semi- semi-automatic carton Overprinting Machines Objective: To lay down a procedure for Operation, Cleaning, and Overprinting of Semi-Automatic Carton Overprinting Machines. Scope: This Standard Operating Procedure is applicable for Operation, Cleaning, and Overprinting Record of Semi-Automatic Carton Overprinting Machine & Semi-Automatic Carton Overprinting Machine. Responsibility Packing Material Store and production…