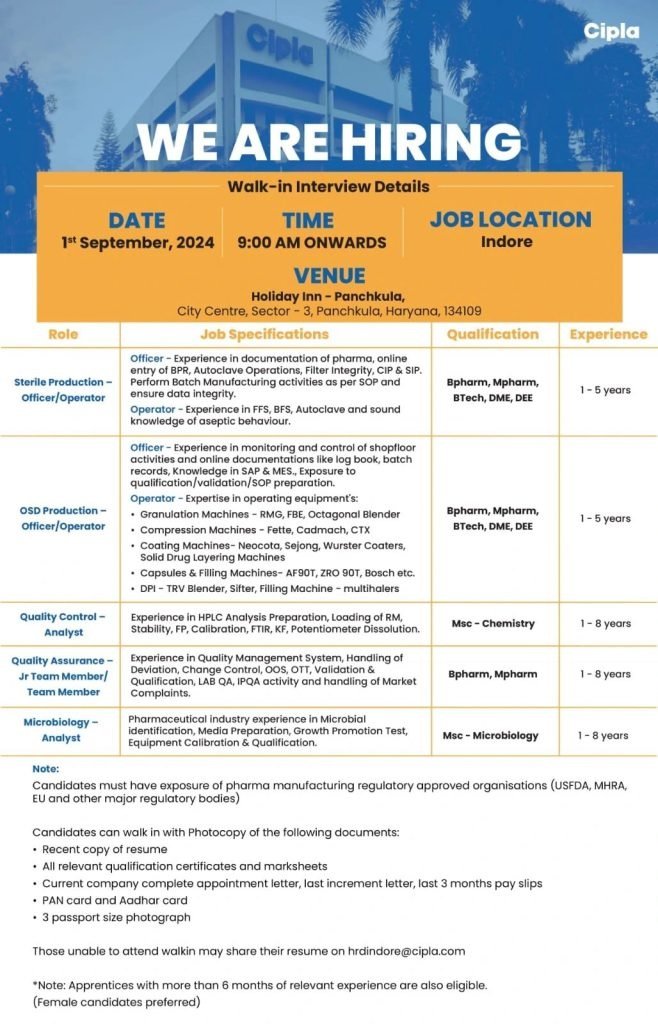
Cipla -Interview for Production, Quality Control, Quality Assurance& Microbiology on1st September 2024
Cipla’s Growing Team! We are excited to invite talented professionals to our walk-in interview event on 1st September 2024 at the Holiday Inn, Panchkula.
Open Positions:
- Sterile Production – Officer/Operator
- Experience: Officer – 1-5 years, Operator – 1-5 years
- Qualifications: BPharm, MPharm, BTech, DME, DEE
- Responsibilities:
- Officer: Documentation, Batch Manufacturing as per SOP, data integrity, Autoclave Operations, Filter Integrity, CIP & SIP.
- Operator: Operating FFS, BFS, Autoclave, and knowledge of aseptic behavior.
- OSD Production – Officer/Operator
- Experience: Officer – 1-5 years, Operator – 1-5 years
- Qualifications: BPharm, MPharm, BTech, DME, DEE
- Responsibilities:
- Officer: Monitoring shop floor activities, online documentations, SAP & MES knowledge, exposure to qualification/validation/SOP preparation.
- Operator: Expertise in operating Granulation, Compression, and Coating Machines.
- Quality Control – Analyst
- Experience: 1-8 years
- Qualifications: MSc Chemistry
- Responsibilities: HPLC Analysis, Stability Testing, Calibration, Microbial Identification.
- Quality Assurance – Jr Team Member/Team Member
- Experience: 1-8 years
- Qualifications: BPharm, MPharm
- Responsibilities: Handling Deviation, Change Control, OOS, OTT, Validation & Qualification, LAB QA, IPQA activity.
- Microbiology – Analyst
- Experience: 1-8 years
- Qualifications: MSc Microbiology
- Responsibilities: Microbial Identification, Media Preparation, Growth Promotion Test, Equipment Calibration & Qualification.
Date: 1st September 2024
Time: 9:00 AM Onwards
Venue: Holiday Inn – Panchkula, City Centre, Sector-3, Panchkula, Haryana, 134109
Note:
- Candidates must have exposure to pharma manufacturing regulatory approved organizations (USFDA, MHRA, EU, etc.).
- Female candidates preferred.
- Apprentices with more than 6 months of relevant experience are also eligible.
Documents to Bring:
- Recent resume
- Relevant qualification certificates and marksheets
- Current company appointment letter, last increment letter, last 3 months pay slips
- PAN card and Aadhar card
- 3 passport-sized photographs
Unable to attend? Share your resume with us at hrdindore@cipla.com.
Job Category: pharma
Job Type: Full Time
Job Location: Indore