Biophore, an established pharmaceutical company, is engaged in the development and manufacturing of niche pharmaceutical products for the generic industry. Within a decade since inception in 2007, Biophore has emerged as a trusted partner in the generic industry across US, Europe and other regulated markets.
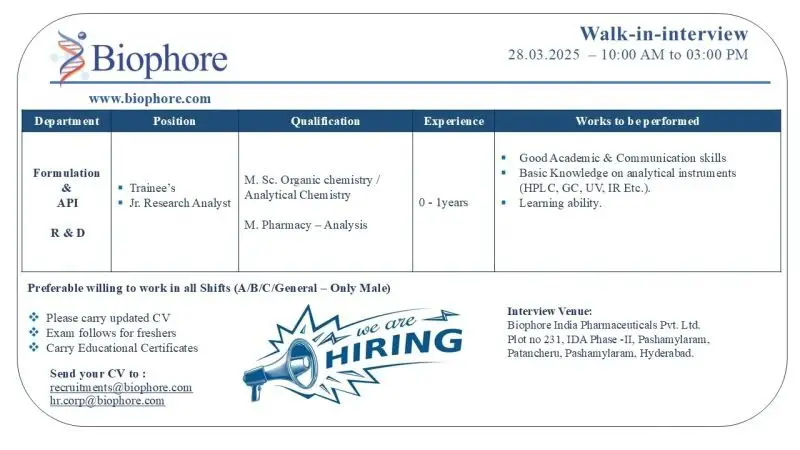
Post : Trainee’s ; Jr. Research Analyst
Department : Formulation & API R&D
Qualification : M. Sc. Organic chemistry / Analytical Chemistry
M.Pharmacy – Analysis
Experience : 0-1 years
Works to be performed : • Good Academic & Communication skills
• Basic Knowledge on analytical instruments (HPLC. GC.UV, IREtc.).
• Learning ability.
Preferable willing to work in all Shifts (A/B/C/General – Only Male)
Please carry updated CV
Exam follows for freshers
Carry Educational Certificates
Walk in Interview
Date : 28-03-2025
Time : 10:00 am to 03:00 pm
Venue : Biophore India Pharmaceuticals Pvt. Ltd. Plot no 231, IDA Phase -II, Pashamylaram, Patancheru, Pashamylaram, Hyderabad.