Aspiro – Interview For Production/ QA/ QC/ Microbiology on 29th Jun 2024
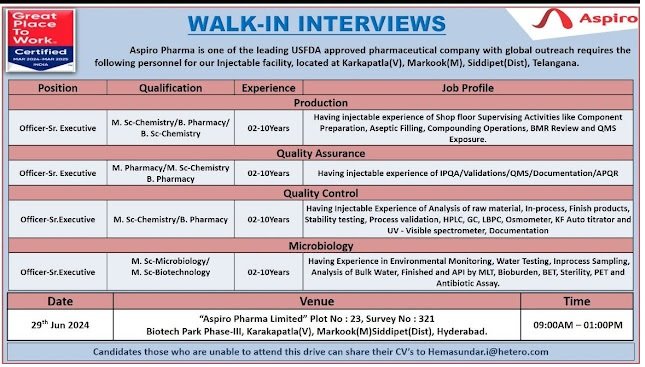
Production
Officer-Sr. Executive
M. Sc-Chemistry/B. Pharmacy/ B.Sc Chemistry
02-10Years
Job Profile
Having injectable experience of Shop floor Supervising Activities like Component Preparation, Aseptic Filling, Compounding Operations, BMR Review and QMS
Quality Assurance
Officer-Sr. Executive
M. Pharmacy/M. Sc-Chemistry B. Pharmacy
02-10Years
Exposure.
Having injectable experience of IPQA/Validations/QMS/Documentation/APQR
Officer-Sr.Executive M. Sc-Chemistry/B. Pharmacy
02-10 Years
Having Injectable Experience of Analysis of raw material, In-process, Finish products, Stability testing, Process validation, HPLC, GC, LBPC, Osmometer, KF Auto titrator and UV-Visible spectrometer, Documentation
Microbiology
M. Sc-Microbiology/ M. Sc-Biotechnology
02-10 Years
Having Experience in Environmental Monitoring, Water Testing, Inprocess Sampling, Analysis of Bulk Water, Finished and API by MLT, Bioburden, BET, Sterility, PET and Antibiotic Assay.
Date-29th Jun 2024
Venue-“Aspiro Pharma Limited” Plot No: 23, Survey No: 321
Biotech Park Phase-III, Karakapatla(V), Markook(M)Siddipet(Dist), Hyderabad.
Time-09:00AM – 01:00PM
Candidates those who are unable to attend this drive can share their CV’s to Hemasundar.i@hetero.com