Ami Lifesciences – Interview For QC and ADL Dept on 2nd March 2024
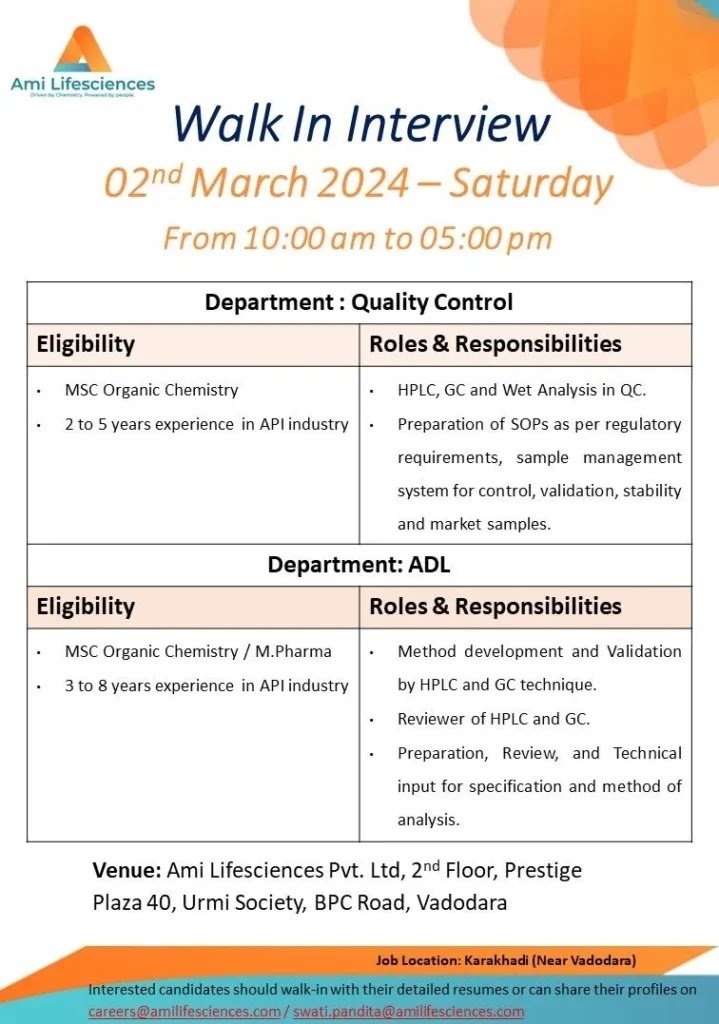
Ami Lifesciences Walk In Interview 02nd March 2024-Saturday
From 10:00 am to 05:00 pm
Department: Quality Control
Eligibility-MSC Organic Chemistry
2 to 5 years experience in API industry
Roles & Responsibilities
HPLC, GC and Wet Analysis in QC.
Preparation of SOPs as per regulatory requirements, sample management
system for control, validation, stability and market samples.
Department: ADL
Roles & Responsibilities
Eligibility-MSC Organic Chemistry / M.Pharma
3 to 8 years experience in API industry
Method development and Validation
by HPLC and GC technique.
Reviewer of HPLC and GC.
Preparation, Review, and Technical
input for specification and method of analysis.
Venue: Ami Lifesciences Pvt. Ltd, 2nd Floor, Prestige
Plaza 40, Urmi Society, BPC Road, Vadodara
Job Location: Karakhadi (Near Vadodara)
Interested candidates should walk-in with their detailed resumes or can share their profiles on careers@amilifesciences.com/swati.pandita@amilifesciences.com

