Alembic Pharma- Walk in Drive for D.Pharm, B.Pharm, M.Pharm, MSc in Production, Packing, QC on 6th July 2025
Alembic Pharmaceuticals Limited’s rich legacy spanning over a century in the Indian Pharma Industry. With a steadfast commitment to quality and innovation, we continue to make significant strides in the pharmaceutical sector.
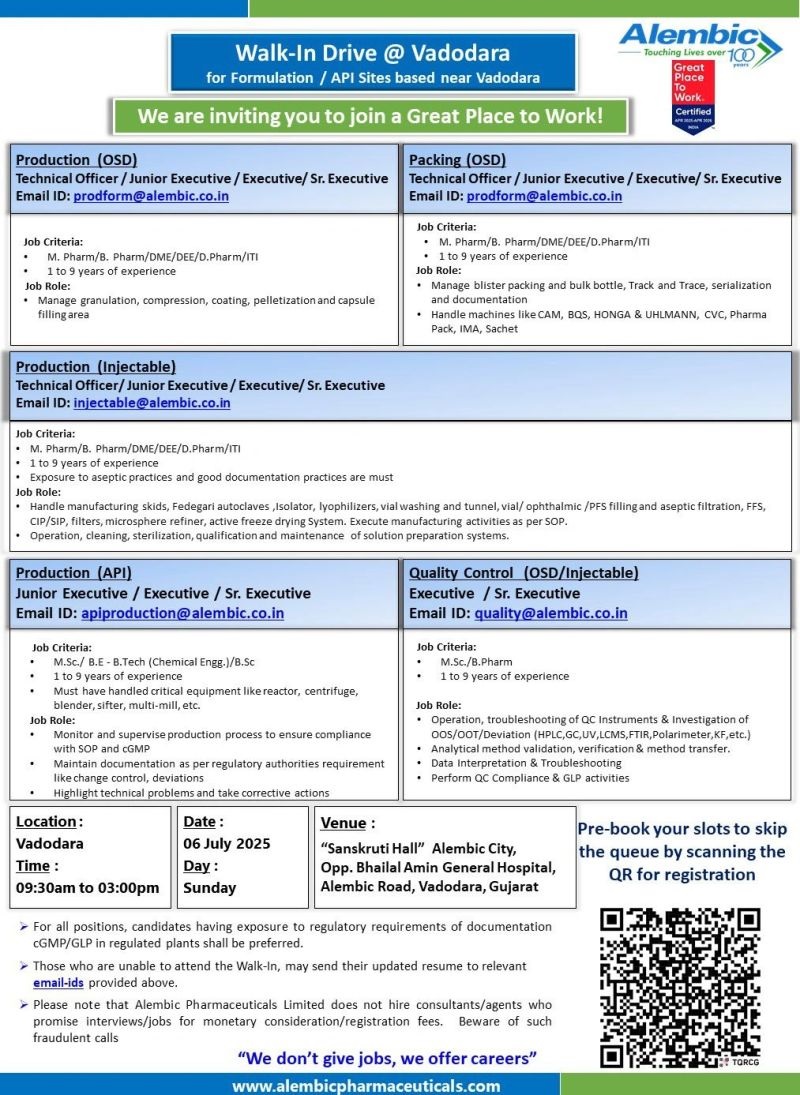
Walk-In Drive at Vadodara
Production (OSD)
Post : Technical Officer/Junior Executive / Executive/ Sr. Executive
Email ID : [email protected]
Job Criteria :M.Pharm / B.Pharm / DME / DEE / D.Pharm / ITI
• 1 to 9 years of experience
Job Role :
• Manage granulation, compression, coating, pelletizationand capsule fillingarea
Packing (OSD)
Post : Technical Officer / Junior Executive / Executive/ Sr. Executive
Email ID : [email protected]
Job Criteria : M.Pharm/B. Pharm/DME/DEE/D.Pharm/lTI
• 1 to 9 years of experience
Job Role :
• Manage blister packing and bulk bottle, Track and Trace, serialization and documentation
• Handle machines like CAM, BQS, HONGA & UHLMANN, CVC, Pharma Pack, IMA, Sachet
Production (Iniectable)
Post : Technical Officer/ Junior Executive/ Executive/ Sr. Executive
Email ID : [email protected]
Job Criteria • M.Pharm / B.Pharm /DME/DEE/D.Pharm/lTI
• 1 to 9 years of experience
• Exposure to aseptic practices and good documentation practices are must Job Role:
• Handle manufacturing skids, Fedegari autoclaves isolator, lyophilizers, vial washing and tunnel, vial/ophthalmic/PFS fillingand aseptic filtration, FFS, CIP/SIP, filters, microsphere refiner, active freeze drying System. Execute manufacturing activities as per SOP.
• Operation, cleaning, sterilization, qualification and maintenance of solution preparation systems.
Production (API)
Post : Junior Executive / Executive / Sr. Executive
Email ID : [email protected]
Job Criteria :
• M.Sc./ B.E – B.Tech (Chemical Engg.)/B.Sc
• 1 to 9 years of experience
• Must have handled critical equipment like reactor, centrifuge, blender, sifter, multi-mill, etc.
Job Role :
• Monitor and supervise production process to ensure compliance with SOP and cGMP
• Maintain documentation as per regulatory authorities requirement like change control, deviations
• Highlight technical problems and take corrective actions
Quality Control (OSD/lniectable)
Post : Executive / Sr. Executive
Email ID : [email protected]
Job Criteria :M.Sc / B.Pharm
• 1 to 9 years of experience
Job Role :
• Operation, troubleshooting of QC Instruments & Investigation of OOS/OOT/Deviation (HPLC,GC,UV,LCMS,FTIR,Polarimeter,KF,etc.)
• Analytical method validation, verification & method transfer.
• Data Interpretation & Troubleshooting
• Perform QC Compliance & GLP activities
Job Location : Formulation / API Sites based near Vadodara
Walk in Interview
Date : 06th July 2025 (Sunday)
Time : 09:30 am to 03:00 pm
Venue : “Sanskruti Hall” Alembic City, Opp. Bhailal Amin General Hospital, Alembic Road, Vadodara, Gujarat
For all positions, candidates having exposure to regulatory requirements of documentation cGMP/GLP in regulated plants shall be preferred.
Those who are unable to attend the Walk-In, may send their updated resume to relevant email-ids provided above.