Assigning Manufacturing and Expiry date to the Finished Products
Objective
To lay down a procedure for assigning manufacturing and expiry dates to the finished products manufactured.
Scope
This procedure shall apply to all products manufactured.
Responsibility
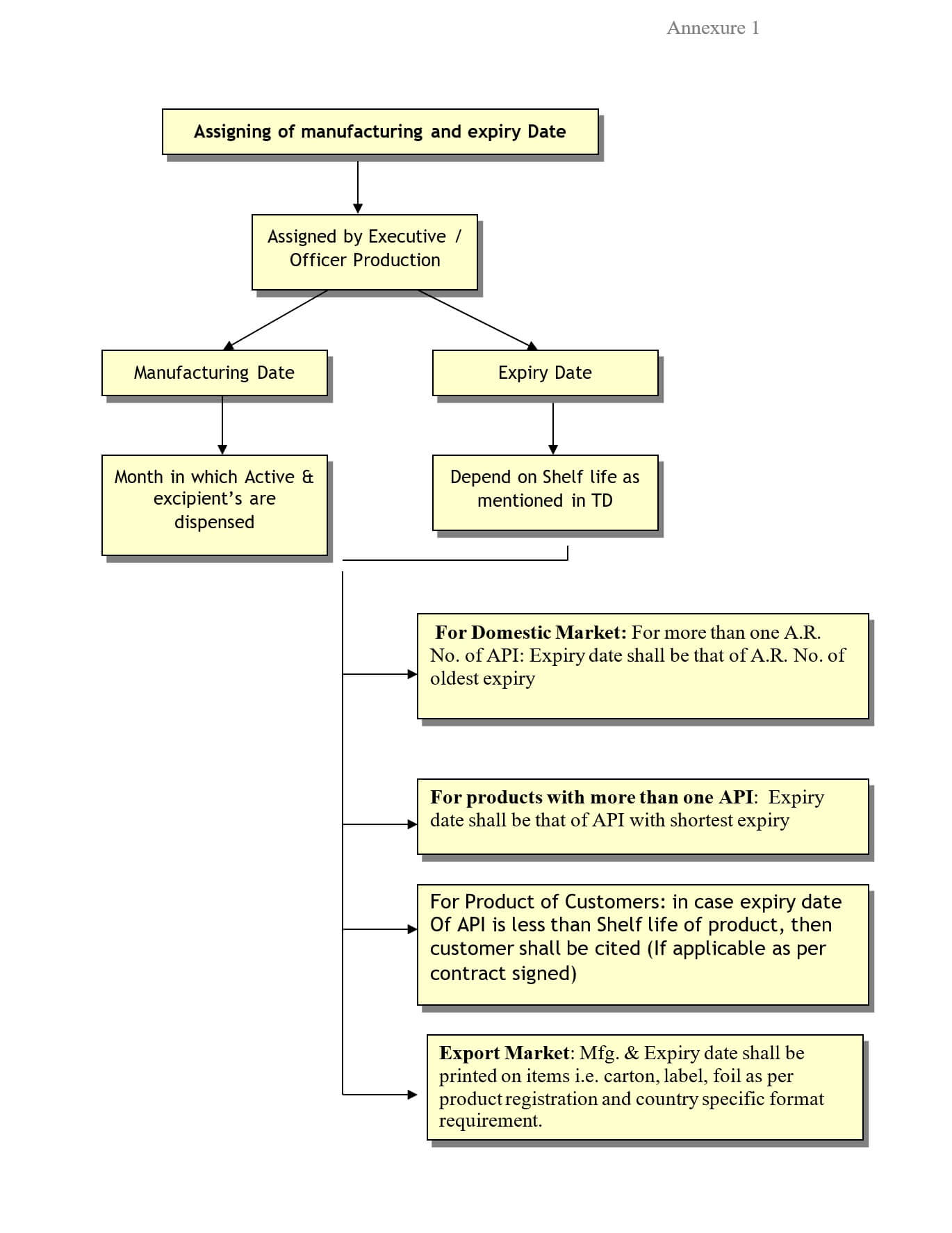
Executive / Officer Production shall be responsible for assigning manufacturing and expiry dates to batches of finished product
Head, of the Production department shall be responsible for the implementation of this SOP.
The head of quality Assurance shall be responsible for compliance of this SOP.
Abbreviations and Definitions
Manufacturing date: The manufacturing date is the month in which raw materials (active and excipients) are dispensed for manufacturing of the product.
Expiry date: It is the date till which a finished product is expected to remain within its approved finished product specifications.
API: Active Pharmaceutical Ingredient
A.R. No. : Analytical Reference number
Procedure
Executive / Officer Production shall assign the manufacturing date to the batch of finished product in month and year format,
The manufacturing date for a batch of finished product shall be the month in which the raw materials (active and excipients) of that batch are being dispensed. For example, if the raw materials for a batch of tablets are dispensed in April of the year 2024, then the manufacturing date assigned to the batch shall be ‘April 2024’.
Executive / Officer Production shall assign the expiry date to the batch of finished products based on the manufacturing date of the batch.
The expiry date of the batch shall depend on the shelf life of the product, as prescribed in the technical direction of the batch.
The assigned expiry date shall not be more than the shelf life of the product.
For Domestic market:
If a product has more than one API, then the expiry of the finished product shall be that of the API with the earliest expiry.
For Example: A product formulated with two APIs is manufactured in Jan 2024 and has a shelf life of two years. The expiry of the first API is Dec 2026. The expiry of the second API is Nov 2025. Then the finished product shall be assigned an expiry date as Nov 2025.
When more than one AR No. of API(s) is used, then the assigned expiry date to the finished product shall be of the API with AR No. bearing the shortest shelf life.
For Example: A product formulated with two APIs is manufactured in Jan 2024 and has a shelf life of two years. The expiry of the first API is Dec 2026. The second API is added with two ARs. No(s); one with expiry Mar 2026 and the other with expiry Nov 2025. Then the finished product shall be assigned an expiry date of Nov 2025
Note: The API(s) to be used in batch should have more than 75% of the assigned expiry period available to rationalize the expiry of the Drug product w.r.t. marketing.
In products with more than one API, the assigned expiry date shall be that of the API with the shortest shelf life.
For the export market: The expiry date shall be assigned as per respective regulatory requirements.
Expiry Date shall be printed on the items i.e. Carton, Foil, label, etc. as per the product registration and countries-specific format requirement e.g. DD/MM/YYYY, MM/YY, DD/MM/YY, MM/DD/YY, MM/DD/YYYY, etc.
For Products of customers:- in case the expiry date of the API is less than the shelf life of the Product, then customer approval shall be taken before assigning the expiry date to the Product.
Forms and Records
Annexure 1- Flow Chart
Distribution
Master Copy – Documentation Cell Quality Assurance
Controlled Copy – Production, Stores, Quality Control, Export, Quality Assurance
Annexure 1- Flow Chart