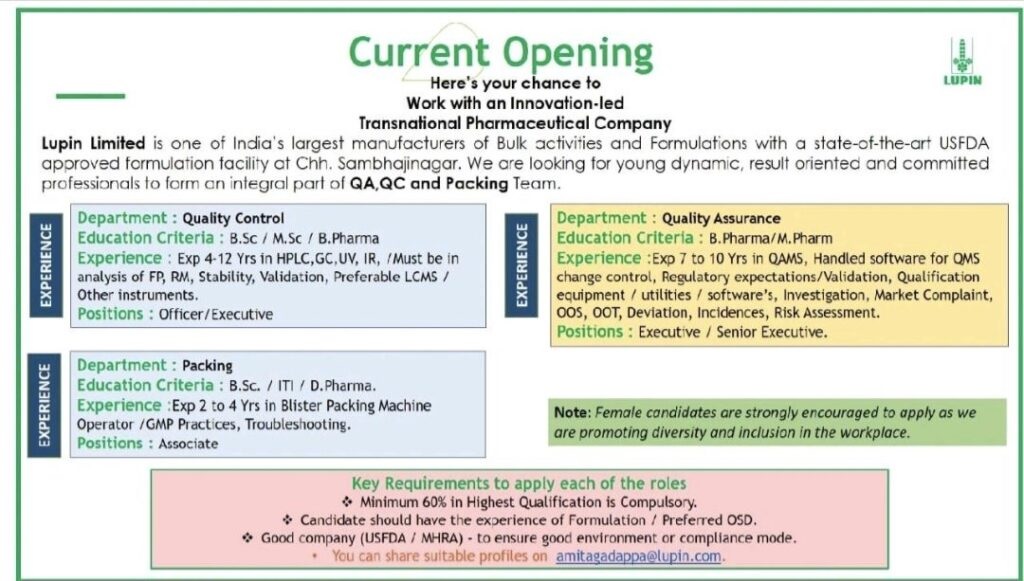
Lupin Limited -Hiring for QA, QC & Packing Roles at USFDA-Approved Plant – Apply Now
Lupin is hiring for multiple roles across Quality Control (QC), Quality Assurance (QA), and the Packing Department. If you have relevant experience and the passion to grow in a regulated environment, these roles might be the perfect fit for you.
1. Quality Control (QC)
Position: Officer / Executive
Qualification: B.Sc / M.Sc / B.Pharma
Experience: 4 to 12 years
Key Skills:
Hands-on experience in HPLC, GC, UV, IR
Analysis of Finished Products (FP), Raw Materials (RM)
Stability, validation activities
Preferably skilled in LCMS and other analytical instruments
2. Quality Assurance (QA)
Position: Executive / Senior Executive
Qualification: B.Pharma / M.Pharma
Experience: 7 to 10 years
Key Responsibilities:
Handling QAMS, QMS software and systems
Change control, validation, qualification of equipment/utilities
Investigation, market complaints, OOS, OOT, deviations, and risk assessment
Ensuring regulatory compliance
3. Packing Department
Position: Associate
Qualification: B.Sc / ITI / D.Pharma
Experience: 2 to 4 years
Skills Required:
Experience with Blister Packing Machines
Good understanding of GMP practices
Ability to perform basic troubleshooting
Eligibility Criteria for All Roles
Minimum 60% marks in the highest qualification is mandatory
Prior experience in formulation (OSD preferred)
Exposure to regulated environments such as USFDA / MHRA is highly desirable
Diversity & Inclusion
Lupin Limited strongly promotes diversity and inclusion in the workplace. Female candidates are especially encouraged to apply and grow with the organization.
Interested candidates can send their updated profiles to:
📧 [email protected]