Indoco Remedies-Interview for M.Pharm, B.Pharm, MSc in Production, QA, QC on 28/09/2025
A leading pharma Company with a strong global presence and international regulatory approvals including USFDA and UKMHRA
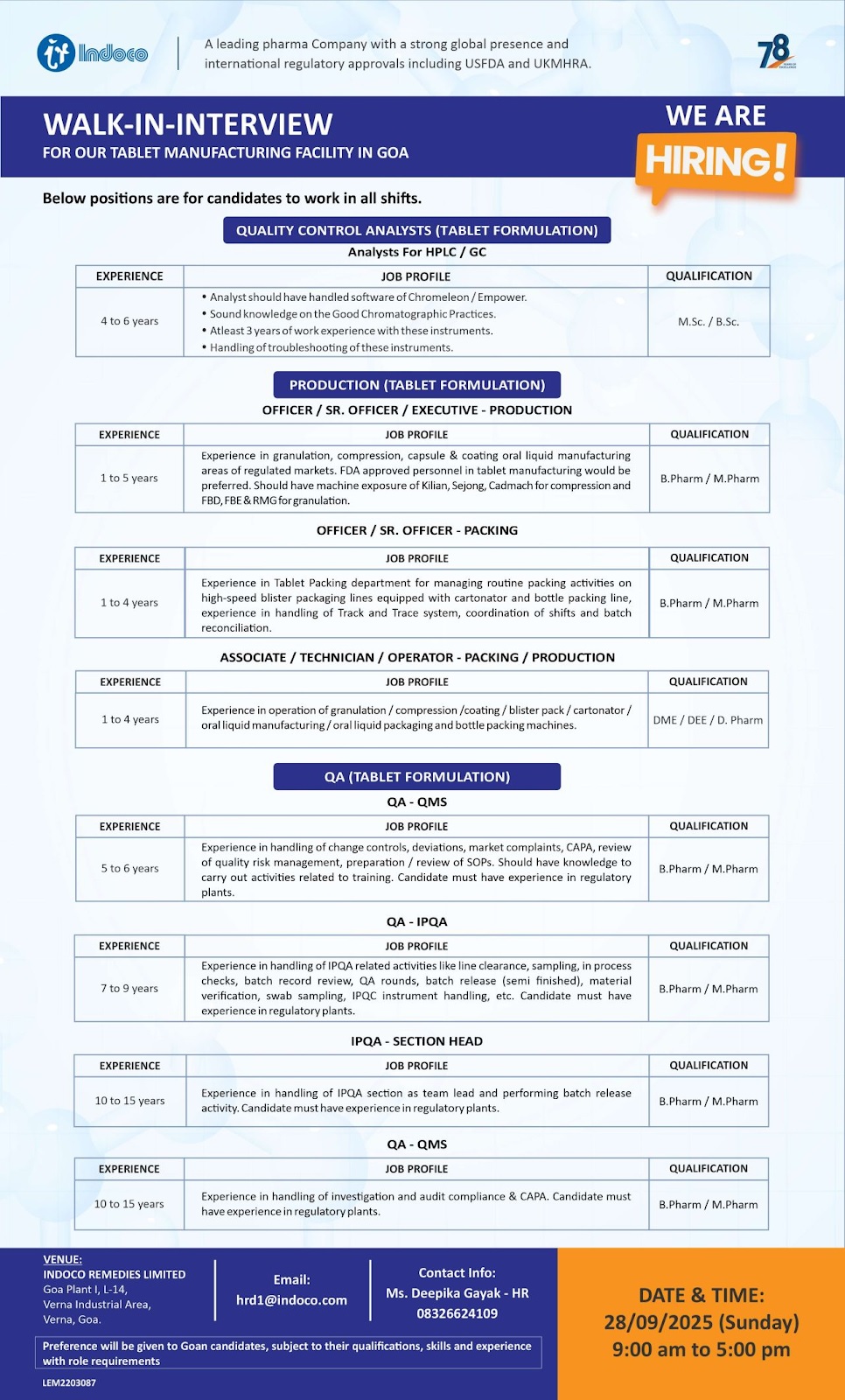
WALK-IN-INTERVIEW FOR TABLET MANUFACTURING FACILITY IN GOA Below positions are for candidates to work in all shifts.
Quality Control Analysts (Tablet Formulation)
Analysts For HPLC / GC
Experience : 4 to 6 years
Job Profile : • Analyst should have handled software of Chromeleon / Empower.
• Sound knowledge on the Good Chromatographic Practices.
• Atleast 3 years of work experience with these instruments.
• Handling of troubleshooting of these instruments.
Qualification : M.Sc. / B.Sc.
Production (Tablet Formulation)
Officer / Sr. Officer / Executive – Production
Experience : 1 to 5 years
Job Profile : Experience in granulation, compression, capsule & coating oral liquid manufacturing areas of regulated markets. FDA approved personnel in tablet manufacturing would be preferred. Should have machine exposure of Kilian, Sejong, Cadmach for compression and FBD,FBE&RMG for granulation.
Qualification : B.Pharm / M.Pharm
Officer / Sr. Officer – Packing
Experience : 1 to 4 years
Job Profile : Experience in Tablet Packing department for managing routine packing activities on high-speed blister packaging lines equipped with cartonator and bottle packing line, experience in handling of Track and Trace system, coordination of shifts and batch reconciliation.
Qualification : B.Pharm / M.Pharm
Associate / Technician / Operator – Packing / Production
Experience : 1 to 4 years
Job Profile : Experience in operation of granulation / compression /coating / blister pack / cartonator / oral liquid manufacturing/oral liquid packaging and bottle packing machines.
Qualification : D.Pharm
QA (Tablet Formulation)
QA- QMS
Experience : 5 to 6 years
Job Profile : Experience in handling of change controls, deviations, market complaints, CAPA, review of quality risk management, preparation / review of SOPs. Should have knowledge to carry out activities related to training. Candidate must have experience in regulatory plants.
Qualification : B.Pharm / M.Pharm
QA – IPQA
Experience : 7 to 9 years
Job Profile : Experience in handling of IPQA related activities like line clearance, sampling, in process checks, batch record review, QA rounds, batch release (semi finished), material verification, swab sampling, IPQC instrument handling, etc. Candidate must have experience in regulatory plants.
Qualification : B.Pharm / M.Pharm
QA – Section Head
Experience : 10 to 15 years
Job Profile : Experience in handling of IPQA section as team lead and performing batch release activity. Candidate must have experience in regulatory plants.
Qualification : B.Pharm / M.Pharm
QA – QMS
Experience : 10 to 15 years
Job Profile : Experience in handling of investigation and audit compliance & CAPA. Candidate must have experience in regulatory plants.
Qualification : B.Pharm / M.Pharm
Walk in Interview
Date : 28/09/2025 (Sunday)
Time : 9:00 am to 5:00 pm
Venue : INDOCO REMEDIES LIMITED Goa Plant I, L-14,
Verna Industrial Area, Verna, Goa.
Preference will be given to Goan candidates, subject to their qualifications, skills and experience with role requirements.