Ipca Laboratories Limited-interview for QA, QC, Engineering, Mfg. (Tablet/ Injection) on 2nd June, 2024
WALK-IN INTERVIEW For Ipca Laboratories Limited, Ratlam (M.P.)
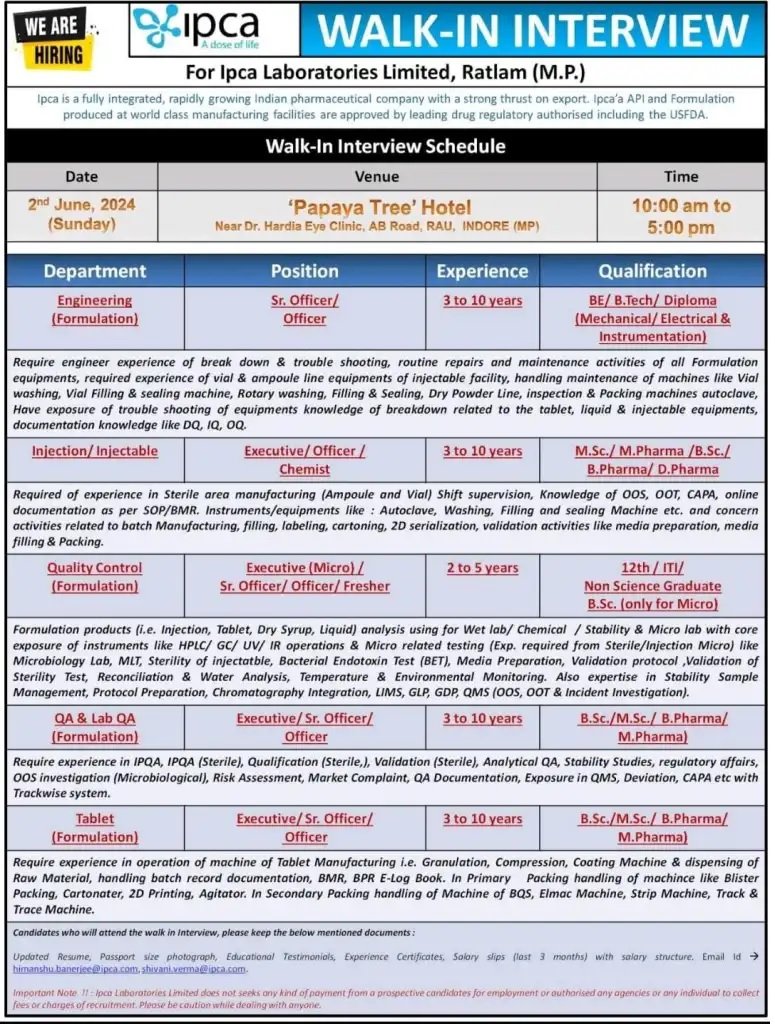
Ipca is a fully integrated, rapidly growing indian pharmaceutical company with a strong thrust on export. Ipca’a API and Formulation produced at world class manufacturing facilities are approved by leading drug regulatory authorised including the USFDA.
Engineering (Formulation)
Position: Sr. Officer/ Officer
Experience required: 3 to 10 years
Require engineer experience of break down & trouble shooting, routine repairs and maintenance activities of all Formulation equipments, required experience of vial & ampoule line equipments of injectable facility, handling maintenance of machines like Vial washing, Vial Filling & sealing machine, Rotary washing, Filling & Sealing, Dry Powder Line, inspection & Packing machines autoclave, Have exposure of trouble shooting of equipments knowledge of breakdown related to the tablet, liquid & injectable equipments, documentation knowledge like DQ, IQ, OQ
Injection/ Injectable
Position: Executive/ Officer/ Chemist
Experience required: 3 to 10 years
Required of experience in Sterile area manufacturing (Ampoule and Vial) Shift supervision, Knowledge of OOS, OOT, CAPA, online documentation as per SOP/BMR. Instruments/equipments like Autoclave, Washing, Filling and sealing Machine etc. and concern activities related to batch Manufacturing, filling, labeling, cartoning, 2D serialization, validation activities like media preparation, media filling & Packing.
Quality Control (Formulation)
Position: Executive (Micro)/ Sr. Officer/Officer/Fresher
Experience required: 2 to 5 years
Formulation products (i.e. Injection, Tablet, Dry Syrup, Liquid) analysis using for Wet lab/Chemical / Stability & Micro lab with core exposure of instruments like HPLC/GC/ UV/IR operations & Micro related testing (Exp. required from Sterile/Injection Micro) like Microbiology Lab, MLT, Sterility of injectatble, Bacterial Endotoxin Test (BET), Media Preparation, Validation protocol, Validation of Sterility Test, Reconciliation & Water Analysis, Temperature & Environmental Monitoring. Also expertise in Stability Sample Management, Protocol Preparation, Chromatography Integration, LIMS, GLP, GDP, QMS (OOS, OOT & Incident Investigation).
QA & Lab QA (Formulation)
Position: Executive/Sr. Officer/ Officer
Experience: 3 to 10 years
Require experience in IPQA, IPQA (Sterile), Qualification (Sterile,), Validation (Sterile), Analytical QA, Stability Studies, regulatory affairs, OOS investigation (Microbiological), Risk Assessment, Market Complaint, QA Documentation, Exposure in QMS, Deviation, CAPA etc with Trackwise system.
Tablet (Formulation)
Position: Executive/Sr. Officer/ Officer
Experience required: 3 to 10 years
Require experience in operation of machine of Tablet Manufacturing i.e. Granulation, Compression, Coating Machine & dispensing of Raw Material, handling batch record documentation, BMR, BPR E-Log Book. In Primary Packing handling of machince like Blister Packing, Cartonater, 2D Printing, Agitator. In Secondary Packing handling of Machine of BQS, Elmac Machine, Strip Machine, Track & Trace Machine.
Interview Details:
Date: 2nd June, 2024 (Sunday)
Time: 10:00 am to 5:00 pm
Venue: ‘Papaya Tree’ Hotel Near Dr. Hardia Eye Clinic, AB Road, RAU, INDORE (MP)
Candidates who will attend the walk in Interview, please keep the below mentioned documents:
Updated Resume, Passport size photograph, Educational Testimonials, Experience Certificates, Salary slips (last 3 months) with salary structure. Email Id→
himanshu.banerjee@ipca.com.
shivani.verma@ipca.com
