STABILITY TESTING OF NEW DRUG PRODUCTS Q1A(R2) Drug Product General Photostability Testing Selection of Batches Container Closure System Specification Testing Frequency Storage Conditions Stability Commitment Evaluation Statements/Labeling Drug Product General The design of the formal stability studies for the drug product should be based on knowledge of the behavior and …
Read More »STABILITY TESTING OF NEW DRUG SUBSTANCES Q1A(R2)
STABILITY TESTING OF NEW DRUG SUBSTANCES Q1A(R2) COVER NOTE FOR REVISION OF Q1A(R) The changes made in Q1A(R) that result from adoption of ICH Q1F “Stability Data Package for Registration Applications in Climatic Zones III and IV”. These changes are: The intermediate storage condition has been changed from 30°C ± …
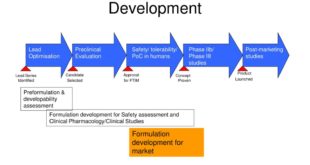
Read More »Pharmaceutical Development as per USFDA
PHARMACEUTICAL DEVELOPMENT I. INTRODUCTION II. PHARMACEUTICAL DEVELOPMENT A. Components of the Drug Product 1. Drug Substance 2. Excipients B. Drug Product 1. Formulation Development 2. Overages 3. Physicochemical and Biological Properties C. Manufacturing Process Development D. Container Closure System E. Microbiological Attributes F. Compatibility III. GLOSSARY INTRODUCTION The Pharmaceutical …
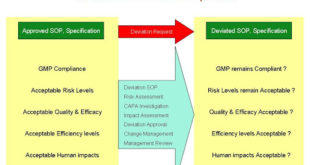
Read More »Deviation Handling and Quality Risk Management
Cleaning Validation as per APIC Guideline
PPT on prevent the spread of COVID 19 infection at the workplace
Investigating Out-of-Specification
Investigating Out-of-Specification IDENTIFYING AND ASSESSING OOS TEST RESULTS — PHASE I: LABORATORY INVESTIGATION PHASE II: FULL-SCALE OOS INVESTIGATION
Read More »INPROCESS CONTROL OF CAPSULE MANUFACTURING
INPROCESS CONTROL OF CAPSULE MANUFACTURING OBJECTIVE: To lay down the procedure for In process Control of Capsule Manufacturing. SCOPE: This SOP covers the responsibility and procedure for In process control during capsule manufacturing. RESPONSIBILITY: In process Quality Assurance Executive/Officer. ACCOUNTABILITY: Quality Assurance Manager. PROCEDURE: Carry out line clearance at each …
Read More »PROCEDURE FOR CALIBRATION OF REFRIGERATOR
OBJECTIVE:To describe the calibration procedure for distribution of temperature within the chamber of Refrigerator. SCOPE: his SOP shall be applicable for Calibration of Refrigerator at Pharmaceutical Industries. RESPONSIBILITY : Quality Control Executive/Officer. ACCOUNTABILITY Head Quality Control PROCEDURE : Operate the refrigerator as per Standard …
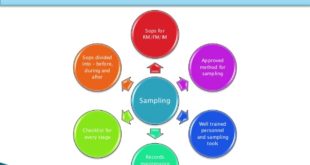
Read More »USFDA GUIDELINE FOR DRUG QUALITY SAMPLING AND TESTING (DQST)- HUMAN DRUGS
USFDA GUIDELINE FOR DRUG QUALITY SAMPLING AND TESTING (DQST)- HUMAN DRUGS
Read More »ANDA Submissions — Content and CTD Format (USFDA)
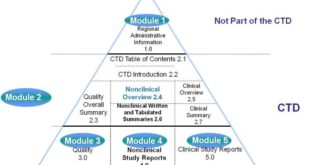
ANDA Submissions — Content and CTD Format (USFDA) TABLE OF CONTENTS CTD FORMAT A. Module 1 – Administrative Information Forms and Cover Letter Administrative Information References Other Correspondence Labeling B.Module 2 – CTD Summaries Quality Overall Summary Clinical Summary C.Module 3 – Quality Drug Substance Drug Product Appendices Regional Information …
Read More »List of Documents required for Pharma Quality Control Departments (GMP & GLP Compliances)
List of Documents required for Store/warehouse (GMP Compliances)
List of Documents required for Quality Assurance Departments (GMP Compliances)
SOP for Operation, Calibration, and Cleaning of the Conductivity meter
1.0 OBJECTIVE:To lay down a Standard Operating Procedure for Operation, Calibration, and Cleaning of the Conductivity meter .2.0 SCOPE:This procedure shall apply to quality control of Pharmaceutical Company for Operation, Calibration, and Cleaning of the Conductivity meter .3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in …
Read More »SOP for Checklist for microbiology data Review.
1.0 OBJECTIVE:To lay down a Standard Operating Procedure for Checklist for microbiology data Review .2.0 SCOPE:This procedure shall apply to quality Control(Microbiology lab) of Pharmaceutical Company for microbiology data Review .3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department Heads shall be …
Read More »SOP for Checklist for Analytical Raw Data Review (Chemical)
1.0 OBJECTIVE:To lay down a Standard Operating Procedure for Checklist for Analytical Raw Data Review (Chemical). 2.0 SCOPE:This procedure shall apply to Quality Ccontrol of Pharmaceutical Company for Analytical Raw Data Review (Chemical) .3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department …
Read More »SOP for Calibration of UV spectrophotometer.
1.0 OBJECTIVE:To lay down a Standard Operating Procedure for Calibration of UV spectrophotometer.2.0 SCOPE:This procedure shall apply for Calibration of UV spectrophotometer in quality control departments .3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department Heads shall be responsible for compliance of …
Read More »SOP for Operation and Calibration of Total Organic Carbon (TOC)
1.0 OBJECTIVE:To lay down a Standard Operating Procedure for Operation and Calibration of Total Organic Carbon (TOC) Analyzer. 2.0 SCOPE:This procedure shall apply for Operation and Calibration of Total Organic Carbon (TOC) Analyzer. .3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department …
Read More »SOP for for Calibration of Gas Chromatography (GC)
Objective: To lay down a procedure for Calibration of Gas Chromatography (GC). Scope: This Standard Operating Procedure is applicable for for Calibration of Gas Chromatography (GC). 3.0 RESPONSIBILITY: All concerned personnel shall responsible to follow the procedure mention in this SOP. Concerned Department Heads shall be responsible for compliance of the procedure. …
Read More »