Personnel Qualification for Aseptic Processing and Sterility Testing Area
Objective:
To lay down the procedure for Personnel Qualification to enter and work in the Aseptic Processing area and Sterility Testing Area.
Scope:
This SOP is applicable for Personnel Qualification to work in Aseptic Processing and Sterility testing Area
Procedure:
For the Aseptic Processing Area
The need for personnel qualification to work in the Aseptic Processing Area shall be identified by the Head-Microbiology Department. in coordination with the Head-Microbiology and Head-Production (Injectables) based on their requirement in the concerned area.
The personnel shall mainly include from production, microbiology, and maintenance departments.
All the identified personnel shall be qualified initially and re-qualified a minimum of once in a year.
The qualification activity shall be performed in two phases.
Phase-1 (Preliminary Qualification): This shall include Classroom training of the person, written test, Gowning demonstration, Trial gowning, and Preliminary monitoring of the person for Viable Count.
Phase-2 (Final Qualification): This shall include observation of person activities in the aseptic processing area and Monitoring of the person for Viable Count after performing the activities in the Aseptic Processing Area. This phase of qualification shall be performed during routine production or process simulation (media fill) studies.
Preliminary Qualification
A person I operator shall undergo classroom training by Head-Microbiology and Head-Injectable Production.
The training shall include the following topics:
Basics of microbiology.
Disinfection and sanitization.
Sterilization.
Personal hygiene.
Aseptic gowning, entry, behavior in aseptic processing area.
Relationship of manufacturing and handling procedures to potential sources of product contaminations.
Consequences of product contamination.
Training procedures may include relevant SOP procedures, Slides, and or PowerPoint presentations.
An attendance record shall be maintained for the training of the person.
After classroom training the person shall be evaluated by a written test, as per a questionnaire (model) given which shall include ten objective-type questions from each relevant topic.
The questions may be changed from person to person and or session to session.
A person shall be given a maximum of three chances to qualify for the written test.
After successful training, the person shall be given a demonstration on aseptic gowning by the Head-Production (Injectable) or Head-Microbiology or their nominees.
After the aseptic gowning demonstration, the person shall undergo trial gowning procedures. The gowning trials shall be observed by Head-Microbiology.
After sufficient practice, the person shall be allowed to enter into change rooms of the aseptic processing area as per SOP No.: (up to changing of sterile gown). The gowning procedure shall be checked by the microbiologist.
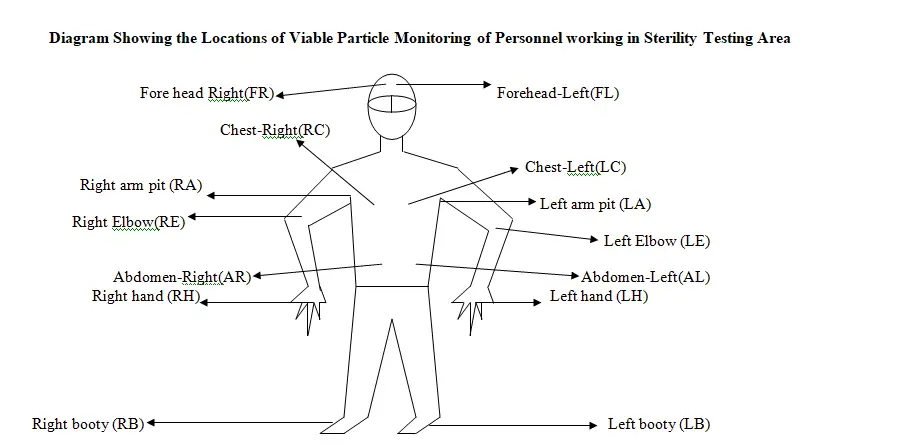
After complete dressing of the sterile gown the person shall be monitored for viable counts in all locations (as per the procedure given in SOP on Viable Particle Monitoring — Sterile Product Manufacturing Facility) by microbiologist using surface contact (RODAC) plates and the person shall be allowed to exit from the exit change rooms.
The viable particle count shall be monitored in all locations (as per the schematic diagram) and the results shall be recorded.
This monitoring shall be repeated for at least three consecutive days (preferably at different times).
The results of this viable monitoring shall be reviewed by Head-Microbiology.
Head—QA Department shall evaluate the Preliminary qualification report and qualify the person for Phase 2.
Final Qualification
After preliminary qualification, the person shall undergo Final qualification to work in an aseptic manufacturing area during media fill trial I routine production.
The person shall be allowed to enter and work in the aseptic manufacturing area for at least two hours.
The activities of the person shall be observed and recorded by both Microbiology and Production (Injectables) Heads.
After each day I shift of activity the person shall be monitored for viable counts for at least four locations (locations of both hands are mandatory) using the procedure followed during preliminary qualification.
The results shall be recorded in the ‘Personnel Monitoring Report given in SOP on Viable Particle Monitoring — Sterile Product Manufacturing Facility’.
The monitoring shall be performed at least three consecutive days, for final qualification.
The results of this viable monitoring shall be reviewed by the Head-Microbiology and attached to the ‘Final Qualification Report’.
The final Qualification report shall be evaluated by the Head-QC Department and approved by the Head-QA Department.
Head—Microbiology shall intimate the qualification result to the person who has undergone the qualification tests and shall receive the signature on a duplicate copy of the intimation.
Copy of intimation shall be sent to Head-Production (Injectables)
All the documents generated during personnel qualification shall be compiled in the form of file and submitted to QA.
The qualification sequence is represented by a schematic representation.
Acceptance Criteria
The person should score more than 90% of the marks in each written test.
The viable particle monitoring results during preliminary monitoring shall be within the specified levels as mentioned.
The person shall wear and maintain the aseptic gown properly and should work properly.
The viable particle results during monitoring for final qualification shall be within the specified levels mentioned in ‘Personnel Monitoring Report given in SOP on Viable Particle Monitoring — Sterile Product Manufacturing Facility’.
Personnel qualification for the Sterility testing area shall also be done using almost the same procedure with some of the changes mentioned below.
Head-QC shall identify the need for qualification of personnel for the sterility testing area, in coordination with Head-Microbiology.
The personnel shall mainly include from microbiology and maintenance departments.
The qualification activity shall be performed in two phases, following the same procedure mentioned in the qualification for the aseptic processing area.
Classroom training shall be given by Head-Microbiology and the training shall include the following topics Basics of microbiology.
• Disinfection and sanitization.
• Sterilization.
• Personal hygiene.
• Aseptic gowning, entry, behavior in sterility testing area.
• Aseptic testing and sources of contaminations during sterility testing.
After sufficient gowning practice, the person shall be allowed to enter in to change rooms of the sterility testing area following the concern entry and exit SOP (up to changing of sterile gown). The gowning procedure shall be checked by an experienced microbiologist.
After complete dressing of the sterile gown the person shall be monitored for viable counts in all locations (as per the procedure given in SOP on Viable Particle Monitoring — Sterility testing area) by an experienced microbiologist using surface contact (RODAC) plates and the person shall be allowed to exit from the exit change rooms.
For ‘Final qualification’ the person shall be allowed to enter and work in the sterility testing area for at least two hours.
The activities of the person shall be observed and recorded by Head-Microbiology.
After each day I shift of activity the person shall be monitored for viable counts for at least four locations (locations of both hands are mandatory) using the procedure followed during preliminary qualification.
The results shall be recorded in the ‘Personnel Monitoring Report given in SOP on Viable Particle Monitoring — Sterility Testing Area’.
Acceptance Criteria
The person shall get more than 90% of the marks in each written test.
The viable particle monitoring results during preliminary monitoring shall be within the specified levels as mentioned.
The person shall wear and maintain the aseptic gown properly and should work properly.
The viable particle results during monitoring for final qualification shall be within the specified levels mentioned in ‘The Personnel Monitoring Report given in the SOP on Viable Particle Monitoring — Sterility Testing area.
Checklist for Aseptic Processing Area
Activity
Press the door interlocking of the sump, open the door with an elbow, enter, and remove plant uniform and footwear.
Press the door interlocking of the first change room with your elbow and enter
Rinse the hands with Sterillium
Wear the primary garment in the following sequence in the first change room, by taking care to avoid contact with the floor –Cap, Shirt, trousers, and Booties.
Press the door interlocking of the second change room with your elbow and enter
Sanitize the hands with Sterillium
Wear the pre-sterilized hand gloves in the second change room, in a manner that the bare hands shall not touch the outer surface of the gloves
Sanitize the hands with Sterillium.
Press the door interlocking of the third change room with your elbow and enter
Open the garment cubicle and take the bag consisting of a sterile secondary garment
Wear sterile garments in the following sequence, by taking care to avoid contact with the floor.
Headgear without touching outer surface-Boiler suit, Goggles
Ensure that the loose ends of the head cover are tucked inside the boiler suit
Sit on the cross-over bench
Tie up one booty and cross the leg on the other side of the bench
Tie up the other booty without touching the bench by hand and cross over the bench
Discard the empty bag in the waste bin provided in the changing room
Look into the mirror to check the proper gowning and ensure that no body part is exposed
Press the door interlocking of the buffer change room with the elbow and enter
Sanitize the gloved hands with disinfectant wear secondary gloves and disinfect again.
Checklist for Sterility Testing Area Gowning
Activity
Show access card to the interlocking system to enter into change Room-1. Remove the apron, and cap and place street garments in the dedicated cubicle.
Remove the lab slippers and keep them aside. Sanitize the hands with 70% IPA. Press the door interlocking switch of change room-2 with the elbow and enter. Sanitize the Hands with Sterillium.
Wear primary garment in the following sequence in change room-2, by taking care to avoid contact with the floor- Head gear, Jacket, trousers, Booties.
Press the door interlocking switch of change room-3 with the elbow and enter. Sanitize the Hands with 70% IPA.
Switch OFF the UV Light of the garment cubicle and Open the door. Pick and wear a pair of Sterile gloves aseptically. Sanitize the gloved Hands with 70% IPA.
Pick up a bag of Sterile garments containing headgear, and a boiler suit. Open the bag and wear Head gear first, followed by a Boiler suit and booties.
Tie the boiler suit over the belly with the help of the string provided. Close the garment from top to bottom with a zipper. Wear the booties.
Sanitize the Hands with 70% IPA. Pick up and wear goggles from the cubicle. Sanitize the Hands with 70% IPA.
Press the door interlocking switch of change room-4 with the elbow and enter. Check in the mirror for proper gowning.
Press the door interlocking switch with the elbow and enter the Sterility Testing Area.
Put the (√) mark in the appropriate column. Reports attached/Not attached.
Remarks:______________________________________________________________
Result: Qualified/Not qualified to enter and work in aseptic processing/Sterility Testing area.
Requalification Due:_________Evaluated By(Head-QC):________Approved By(Head-QA):_________
PERSONNEL QUALIFICATION FOR ASEPTIC PROCESSING/STERILITY TESTING AREA
Intimation
To
Mr._______________________Designation:_______________ Department:_____________________
Reports attached/Not attached. Remarks:______________________________________________
Result: Qualified/Not qualified to enter and work in aseptic processing/Sterility Testing area.
Requalification Due:____________Evaluated By(Head-QC): ________Approved By(Head-QA):________
Observation of Personnel Activity in the Aseptic Processing Area:
Name of Trainee:______________Designation:_______________Department.:_________________
Activity
Is the Person moving /working in the aseptic processing area slowly and rhythmically?
Is the person disinfecting the hands before and after touching any surface?
Is the person leaning over any sterile material?
Is the person sitting on any surface in the aseptic processing area?
Is the person picking any material lying on the floor?
Is the person sneezing/spitting/coughing in the aseptic processing area?
Is the person keeping all the sterilized materials in a safe place?
Is the person performing any activity, which may alter the sterility of the product?
Is the person laughing or talking in the aseptic processing area?
Observation of Personnel Activity in Sterility Testing Area:
Activity
Is the Person moving /working in the sterility testing Area slowly and rhythmically?
Is the person disinfecting the hands before and after touching any surface?
Is the person leaning over any sterile material?
Is the person sitting on any surface in the Sterility Testing area?
Is the person picking any material lying on the floor?
Is the person sneezing/spitting/coughing in the aseptic processing area?
Is the person keeping all the sterilized materials in a safe place?
Is the person performing any activity, which may alter the sterility of the product?
Is the person laughing or talking in the Sterility Testing Area?
Preliminary Qualification Report
Name of Trainee:______________Designation:_______________Department.:_________________
Tests
All relevant training attendance -Attended/Not Attended
All written tests -Attended/Not Attended
Aseptic Gowning – Proper/Not Proper
Viable Count in Preliminary Qualification-Within Acceptable level/ Not within Acceptable level
Remarks:____________________Result: Qualified/Not qualified for final qualification.
Reviewed By(Head-Microbiology):___________Evaluated By(Head-QC):________________
Report of Viable Particle Count(Preliminary Qualification):
Report of Viable Particle Count (Preliminary Qualification)
Section :____________________Time of test From ____________To____________
Medium Used_______Medium Lot No._______Tested on _______Reported on________
Incubation Temperature(20 – 25oC /30 – 35oC ), Time: From:_____ To:_____
Total viable Count (CFU’s / Contact Plate)
- After 72 hrs. (Bacterial/ Fungal)
- After a further 48 hrs.: (Bacterial/ Fungal)
- Total Viable count after 5 Days ___________
Observation done by______Date of observation__________
Location Name
- Right hand
- Left hand
- Right Chest
- Left Chest
- Fore head-right
- Fore head-Left
- Right armpit
- Left armpit
- Right Elbow
- Left Elbow
- Abdomen-Right
- Abdomen-Left
- Right booty
- Left booty
PERSONNEL QUALIFICATION FOR ASEPTIC PROCESSING/STERILITY TESTING AREA
Written Test
Each Question Carries 1 Mark. Indicate the correct answer with the appropriate alphabet in the box.
Q 1. Microbiology is the study of ______________________________
- A) Microchips
- B) Microfinance
- C) Microorganisms
- D) Microprocessors
Q 2. Bacteria are _________________________________
- A) Eukaryotic organisms
- B) Prokaryotic organisms
- C) Non of the above
- D) Both ‘A’ and ‘B’
Q3. is endospore-forming bacterium ___________________________
- A) Bacillus
- B) Pseudomonas aeruginosa
- C) Escherichia coli
- D) None of the above.
Q 4. Phototrophs require __________________________ as an energy source.
- A) Carbon
- B) Reduced inorganic compounds
- C) Organic compounds
- D) Light
Q 5. Sterilization Means____________________
- A) Process to remove sub-visible particles
- B) Process to remove visual particles
- C) Process to kill or remove insects
- D) Process to kill or remove living microorganisms
Q 6. Sterilization in autoclave is ________________
- A) Chemical sterilization method
- B) Sterilization by filtration
- C) Sterilization by irradiation
- D) Moist heat sterilization
Q 7. Pathogen ____________________________
- A) Produce infectious disease
- B) Doesn’t produce infection
- C) Prevent infection
- D) Reduce infection
Q 8. Bacterial Endotoxins are______________________
- A) Antibiotics
- B) Pyrogenic substances
- C) Antipyretics
- D) Anti-inflammatory agents
Q 9. Bacterial Endotoxins are released from
- A) Fungus’
- B) Virus
- C) Gram -Ve bacteria
- D) Gram +Ve bacteria
Q 10. UV sterilization is an example for _______________________
- A) Chemical sterilization
- B) Dry heat sterilization
- C) Irradiation
- D)Moist Heat Sterilization