SOP For MFR For Ornidazole Tablets 500 mg
Label Сlaim
Manufacturing Formula
List of Equipment
Manufacturing Instructions
Manufacturing Process Flow Chart
Manufacturing Process Details
Packaging
Finished Product Specifications
Procedural Changes
Label Сlaim
| Product | Ornidazole Tablets 500 mg |
| Strength | 500 mg |
| Label сlaim | Each Film uncoated tablet contains:
Ornidazole …………500 mg Brilliant Blue, Quinoline Yellow and Titanium Dioxide IP. |
| Shelf life* | 24 months |
| Storage | Store at temperature below 25° C. Protect from light. |
| Category | Quinolone antibiotic |
* The shelf life is tentative and shall be finalized based on stability studies.
- Manufacturing Procedure .
Bill of Materials:
| Ingredient | Pharmacopoeial Status | Pharmaceutical
role |
| Ornidazole | IH | Active |
| Cellulose, Microcrystalline | Ph.Eur. | Diluent |
| Lactose Monohydrate | Ph.Eur. | Diluent |
| Silica, Colloidal Anhydrous | Ph.Eur. | Surfactant |
| Povidone (K-30) | Ph.Eur. | Binder |
| Water, Purified | Ph.Eur. | Vehicle |
| Sodium Lauril sulphate | Ph.Eur. | Surfactant |
| Talc | Ph.Eur. | Granular former |
| Magnesium Stearate | Ph.Eur. | Lubricant |
| Croscarmellose Sodium | Ph.Eur. | Disintegrating |
| Opadry II (85G51314) Green | IH | Coloring
Agent |
| Water, Purified | Ph.Eur. | Vehicle |
Manufacturing Instructions:
- Ensure machine guards are in place before starting the operations.
- Maintain raw material and finished product at temperature 20 – 25 °C and relative humidity 50 ± 20 %. The same environmental conditions should be maintained during manufacturing.
- The following ‘Personal Protective Measures’ are to be taken: When working with the active substance, drug products or with mixtures of the active substance and excipients wear latex gloves and dust masks to avoid exposure and contact to any body parts.
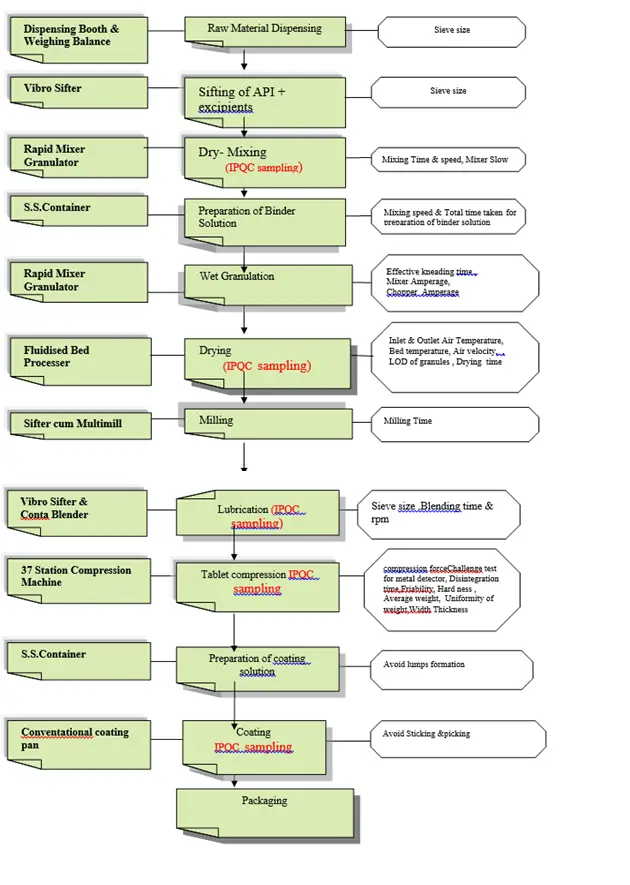
Manufacturing Process Flow Chart
1.0 Manufacturing procedure in brief comprise of following steps:
1.1 Sift, Lactose Monohydrate, Silica, Colloidal Anhydrous, Ornidazole, Cellulose, Microcrystalline through Vibro Sifter fitted with sieve of mesh size 60, and collect the sifted material in IPCs.
1.2 Perform dry mixing of the materials of step (1.1) in Rapid Mixer Granulator. Mix for 5 minutes at mixer slow speed and chopper off.
1.3Prepare binder solution by dissolving Povidone (K-30) in water, purified in S.S.container stir it with the help of mechanical stirrer.
1.4 Preparation of Granules by wet granulation – Add binder solution of step (1.3) to the mixed mass of step (1.2) in RMG and knead for 5 minutes. Add water, purified if required to achieve granulation end point . Record the critical parameters ,Mixer Amperage, Chopper Amperage, Effective kneading time, Extra quantity of Water, purified added , Total quantity of Water, purified consumed.
1.5 Dry the granules of step (1.4) in FBD (with raking at regular intervals) at an inlet air temperature of 50-55°C to a L.O.D of 1.0-2.0% w/w.
Record the critical parameters, Inlet air temperature, Outlet air temperature, Bed temperature, Exhaust flap opening , LOD of granules, Drying time .
1.6 Mill the dried granules of step(1.5) in Sifter cum multimill fitted with screen of 1.5mm for both Sifter and Miller. Collect the granules in blender’s bin & weighing is done by using a calibrated balance.
1.7 Lubrication of Granules
1.7 (a)Sift Talc, Magnesium Stearate ,Sodium Laurilsulfate ,Croscarmellose Sodium through Vibro Sifter fitted with sieve of mesh size 60, and collect the sifted material in IPCs.
1.8 (b) Load the material of step {1.7(a)} in blender’s bin of of step (1.6) and blend it for 10 minutes at 6 rpm.
1.9 Weighing of lubricated granules is done by using a calibrated balance and calculate the final yield..
1.10 Inform to IPQA Department through In-process Analytical Request / Report to collect the sample of blended granules of step (1.8), IPQA personnel send the sample to Quality Control Department for testing as per In-process Specification.
Compression:
1.11 After getting quality control approval take line clearance from IPQA as per respective SOP for Tablet Compression area and all equipment there in to be used for this operation
1.12 After getting approval from IPQA Department, compress the blend of step (1.8), into tablets of required specification using 37 station compression machine fitted with dies and oblong,standard concave punches (17.80 ± 0.05 mm x 75 ± 0.05 mm),with score line on the lower punch. Dedust the tablet by using tablet dedusting machine.
Compression Parameter:
Description: Cream to light yellow coloured ,Oblong, Biconvex, uncoated tablets plain on both side.
Disintegration Time (minutes): NMT 15 at 37°C in water
Friability (% w/w) : NMT 1.0%.
Thickness : 5.0 ± 0.2 mm
Length : 17.80 ± 0.05 mm
Width : 7.75 ± 0.05 mm
Average Weight :686.00 mg to 714.00 mg
Uniformity of weight : Individual weight of 20 Tablets should not deviate more than ±5.0% of the average weight
Hardness: 100-200 N
-
- Carry out in-process control /check as per SOP.
- Weigh and calculate the final yield of core Tablet.
- Inform to IPQA Department through In-process Analytical Request / Report to collect the sample of tablets for analysis as per approved in process specification.
- After getting quality control approval take line clearance from IPQA, coat the Tablets of step (7.1.12),as per required specification.
Coating:
1.13 Preparation of coating dispersion –Take total amount of water, purified in S.S container and stir with mechanical stirrer. Add Opadry II (85G51314) Green slowly & stir the dispersion for 45 minutes, avoid lumps formation and pass through 200 mesh nylon cloth and divide into two equal parts by weight.
1.14 Charge the dedusted tablets into coating pan , heat the core tablets bed with filtered warm air to a temperature 35 – 40oRotate the pan at 1.5 – 8 rpm and spray the coating dispersion, continue with intermittently spraying and drying till required weight build up is achieved. Ensure homogeneity of the coating solution . Dry the tablets completely. Calculate the percentage weight build up for complete batch. Repeat the procedure for second lot.
20 tablets to be collected after every one hour upto percentage weight gain of 2.0% and then after every 15 minutes till the required weight gain (2.0% and 3.0% of average weight of coated tablets) is achieved and tested for Description, Thickness, Length, Width, Disintegration time, Average mass and Uniformity of mass at IPQA lab. Also the composite sample (100 Tablets) shall be collected after completion of coating and submitted to QC for analysis.
In-Process Controls:
Description: Green coloured, oblong, biconvex, film coated tablets plain on both side.
Average mass: 707.0 to 735.0 mg
Uniformity of mass : Individual mass of 20 Tablets should not deviate more than 5.0% of the average mass.
Disintegration Time: Not More Than 30 minutes
Thickness: 5.1 ± 0.20 mm
Length :17.80 ± 0.05 mm
Width :7.75 ± 0.05 mm
1.15 Weigh the coated tablets using calibrated balance and calculated actual yield
1.16 Inform to IPQA Department through In-process Analytical Request / Report to collect the sample of tablets for analysis as per approved in process specification.
1.17 After getting approval from IPQA Department, pack the approved tablets of step 1.15 as per approved specification (Packaging)
Finished Product Specifications
Analyze the finished product samples as per Current Standard Testing Procedure.
Procedural Changes
If a production department has compelling reasons for modifying this manufacturing procedure, it should proceed only after approval from Research & Development and Quality Assurance department.