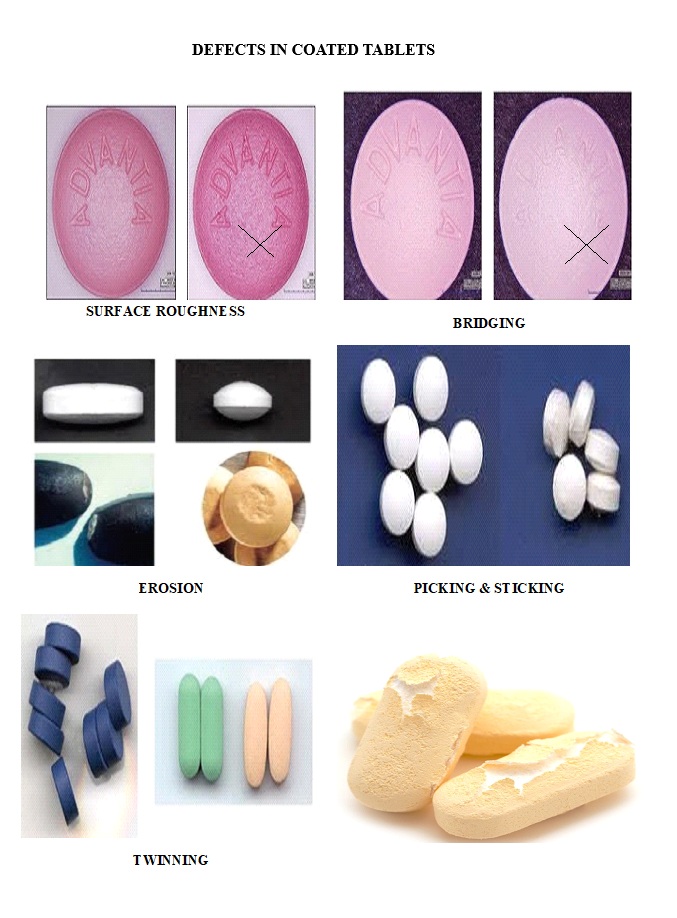
SOP FOR ACCEPTABLE QUALITY LEVEL (AQL) FOR VISUAL INSPECTION
PURPOSE: To lay down a procedure for Acceptable Quality Level (AQL) for visual inspection.
SCOPE: This procedure is applicable for the Acceptable Quality Level (AQL) for visual inspection of Cephalosporin Block.
RESPONSIBILITY:
Preparation of SOPs: Officer/Sr. officer.
Checking and Review of the SOPs: Executive /Sr. Executive.
Approval of the SOPs: Head of Department/ Assistant Manager.
Authorization of SOP: Head QA/ His or her Designee
ACCOUNTABILITY: The accountability of implementation and ensuring compliance of the SOP lies with head of Respective Department.
PROCEDURE:
Acceptable Quality Level (AQL) checks shall be performed semi-finished for commercial batches of tablet & injection.
QA shall perform the Acceptable Quality Level (AQL) checks in the respective area and based on finding.
QA shall decide the need & extend of inspection for the subjected batch and details shall be recorded.
In case of process validation batches, 100% visual inspection shall be performed and the same shall be addressed in the batch record.
During process validation, segregate the visual inspection rejection and evaluate the type of rejection i.e. Critical, Major & Minor.
If the visual inspection trend of process validation is satisfactory, then based on process validation (report) recommendations, Acceptable Quality Level (AQL) sampling shall be performed in commercial batches.
Selection of Containers for Acceptable Quality Level (AQL) sampling of Tablet/Injection:
Production officer shall submit dully filled and signed BMR to QA for review and intimate for visual inspection of the bulk product as per Acceptable Quality Level (AQL).
QA shall ensure Product name, Batch No., Manufacturing date and select the containers of product for Acceptable Quality Level (AQL).
The samples to be withdrawn for the bulk approval from the number of the container shall be based on the following criteria:
Determine the “total number of containers to be sampled” per part lot (for coated tablets) or per batch (for injection & uncoated tablets) by using formula 10+√n+1, where “n” is the total number of containers per part lot/batch.
If the total number of the container is less than or equal to 10 No’s (per batch/lot), then samples shall be checked from all containers.
In case of total number of the container is more than 10, then for Acceptable Quality Level (AQL) sampling of 10 containers shall be done 100% and the remaining container shall be AQL as per formula √n+1. Example: Number of containers is 35 then AQL of 10 (First 5 + Last 5) container is 100% % for remaining (35-10=25) 5 containers, as per formula (√25+1=5+1), 6 containers shall be checked.
If any value of √n is above the whole number, the number shall be rounded off to the next whole number.
For coated tablets, if coating performed in multiple lots, then individual lot size and accordingly Acceptable Quality Level (AQL) samples shall be withdrawn. Example: If 1 part lot contains 28 containers (e.g. coating is performed in two lots) the number of containers to be sampled shall be 10+√18+1 = 10+4.24+1 = 15.24. Therefore, the total number of containers to be sampled shall be 16 from each lot.
If one batch contains 50 containers (e.g. uncoated tablets/injections) then the number of containers to be sampled will be 10+√40+1 = 10+6.32+2 = 17.32. Therefore, the total number of containers to be sampled shall be 18 from the whole batch.
Collection of samples and acceptance criteria for Acceptable Quality Level (AQL):
Collect the samples from each selected containers in equal quantity in a duly labeled polythene bag.
Check each collected tablet (Coated and uncoated) or injection for the quality attributes specified as per Acceptable Quality Level (AQL) on the inspection trolley.
Example: In case of uncoated tablets/capsules, if Acceptable Quality Level (AQL) sample quantity requirement is 1250 and no. of containers to be sampled is 9 then from each container 1250/9 i.e. 138.9 = 139 samples (Withdrawn of samples = No. of sample * Average weight of sample by manual counting) shall be withdrawn and composite sample of 1250 bulk unit shall be prepared.
In case of coated tablets if the coating is performed in two lots and lot size is 500000 then 800 tablets from each part lot shall be withdrawn.
If samples are to be withdrawn from 5 containers, then from each container 800/5 = 160 coated tablets shall be withdrawn.
For the purpose of the Acceptable Quality Level (AQL), consider the sampling standard weight of tablets as average weight.
The acceptance criteria for Acceptable Quality Level (AQL) shall be given below based on classification of the defects:
| Defect Class | Definition | AQL |
| Critical | A defect that can compromise product safety, purity, or identity that may be harmful to the consumer. | 0.1% |
| Major | A defect that jeopardizes the integrity or function of the package. | 1.5% |
| Minor | A defect that does not affect product safety, purity, or identity, or package integrity of function. | 2.5% |
The sample quantity for bulk approval and number of defects observed against acceptance criteria to determine whether batch passes or fails shall be as per below table:
| Sample size code letter | Total Quantity represents for sampling | No. of sample to be drawn | Acceptance Quality Level | |||||
| Critical (0.1%) | Major (1.5%) | Minor (2.5) | ||||||
| A | 02-08 | 2 | 0 | 1 | 0 | 1 | 0 | 1 |
| B | 09-15 | 3 | 0 | 1 | 0 | 1 | 0 | 1 |
| C | 16-25 | 5 | 0 | 1 | 0 | 1 | 0 | 1 |
| D | 26-50 | 8 | 0 | 1 | 0 | 1 | 0 | 1 |
| E | 51-90 | 13 | 0 | 1 | 0 | 1 | 1 | 2 |
| F | 91-150 | 20 | 0 | 1 | 1 | 2 | 1 | 2 |
| G | 151-280 | 32 | 0 | 1 | 1 | 2 | 2 | 3 |
| H | 281-500 | 50 | 0 | 1 | 2 | 3 | 3 | 4 |
| J | 501-1200 | 80 | 0 | 1 | 3 | 4 | 5 | 6 |
| K | 1201-3200 | 125 | 0 | 1 | 5 | 6 | 7 | 8 |
| L | 3201-10000 | 200 | 0 | 1 | 7 | 8 | 10 | 11 |
| M | 10001-35000 | 315 | 0 | 1 | 10 | 11 | 14 | 15 |
| N | 35001-150000 | 500 | 0 | 1 | 14 | 15 | 21 | 22 |
| P | 150001-500000 | 800 | 0 | 1 | 21 | 22 | 21 | 22 |
| Q | 500001-Over | 1250 | 0 | 1 | 21 | 22 | 21 | 22 |
Note: If batch/part lot fails in Acceptable Quality Level (AQL), acceptance criteria then 100% inspection of the part lot/batch shall be performed.
REFERENCE:
In House