Qualification of existing facilities, systems, equipment, and utilities
An existing facility has a mixture of new fully qualified equipment (DQ, IQ, OQ, PQ) and partially qualified/ unqualified equipment.
Through many years of use, it can usually be shown that facilities, systems, equipment, and utilities can function to varied requirements. Nevertheless, cGMPs necessitate that, items that have an impact on the quality of the intermediate or API, should be qualified.
Formal qualification is the basis for the related activities such as validation associated with new product introduction or validation of existing processes.
Qualification should provide documented verification that the parameters defined as critical for operation and maintenance are adequately controlled.
It is essential that the qualification is practical and achievable, adds value to the project, and is concentrated on the critical elements of the system.
It is recommended that a risk assessment approach is used to define the depth of qualification. There should always be a reasonable proportionality between risk for the product quality, the amount of measures to be taken, and documentation to be prepared (.e.g. stage of manufacture, regulatory status, and intended use)
Regulatory requirements The ICH Q7A
The requirement is that facilities, systems, equipment and utilities are properly qualified and maintained to assure data and product integrity.
PIC/S Guidance:
“While it is not possible to undertake the details of an Installation Qualification for established equipment nor the detailed approach for an Operational Qualification, nevertheless there should be data available that support and verify the operating parameters and limits for the critical variables of the operating equipment.
Additionally, the calibration, cleaning, preventative maintenance, operating procedures and operator training procedures for the use of the equipment should be documented and in use as standard operating procedures (SOPs).”
Risk Assessment
RA is a formal and systematic approach to identify GMP risks related to equipment and supporting systems. It is a very helpful tool that can be applied to plant, equipment, and systems which have been in use for many years.
It is recommended that the manufacturing instructions are used in combination with general GMP requirements (e.g. design, documentation, risk for contamination, usage in final dosage form, etc.) as the basis for the risk assessment.
Each manufacturing step can be assessed individually with respect to critical operations/activities (e.g. stirring speeds, temperature ranges, pressure, hygroscopy, open product handling, etc.).
Process descriptions, development reports, product quality reviews and process validation reports can assist in identifying the quality critical operations and parameters.
Equipment risk assessment can be performed by equipment train or by individual processing units and supporting systems. A grouping strategy of similar/identical processing units can be used.
Unit operations or functions could be assessed according to the following scheme:
- Does Function / Operation directly impact product quality?
- Does Function / Operation create (electronic) data which are the basis for GMP related activities?
- Would malfunction impact directly on product quality?
- Does a measuring instrument control or measure quality critical processing steps/parameters?
- Does Operation / Installation cause contamination risk of product or of the facility environment?
- Are materials of construction in direct contact with product?
- If any of the above questions are answered with „yes“, the operation/function is considered as GMP-relevant. During risk assessment, the probability of occurrence and detectability should be considered and measures to reduce the risk identified.
An example of a technical GMP risk assessment is given below:
The objective of the risk assessment
– Identification of potential risks related to products, preventive/corrective actions, and checks related to qualification.
Scope
– Identification of processing unit and location.
General
– Description of the processing unit (size, configuration, supporting systems (heating/cooling/vacuum/nitrogen/solvents, etc.), type of products produced (e.g. APIs, API intermediates, drug product application, etc.), possible connections with other processing units (like filters, centrifuges, supporting systems, containers, gases, water systems, waste gases, etc.),
System boundaries
– Description of what will be addressed during the risk assessment Related documents/baseline documents for risk assessment.
– P &IDs, engineering documents, SOPs, deviations.
The risk assessment can be performed using a template, where typical working steps /activities for the processing units are listed (including instrumentation and control systems).
An example is given below for a reactor:
– Stirring
– Crystallisation
– Supporting systems
– Heating/cooling
– Charging / discharging
– Evacuation
– Sampling
Additionally, materials of construction with direct product contact are assessed and critical measuring `and control devices are identified that need to be calibrated.
Procedure
It is recommended that the following activities are carried out during qualification of existing equipment:
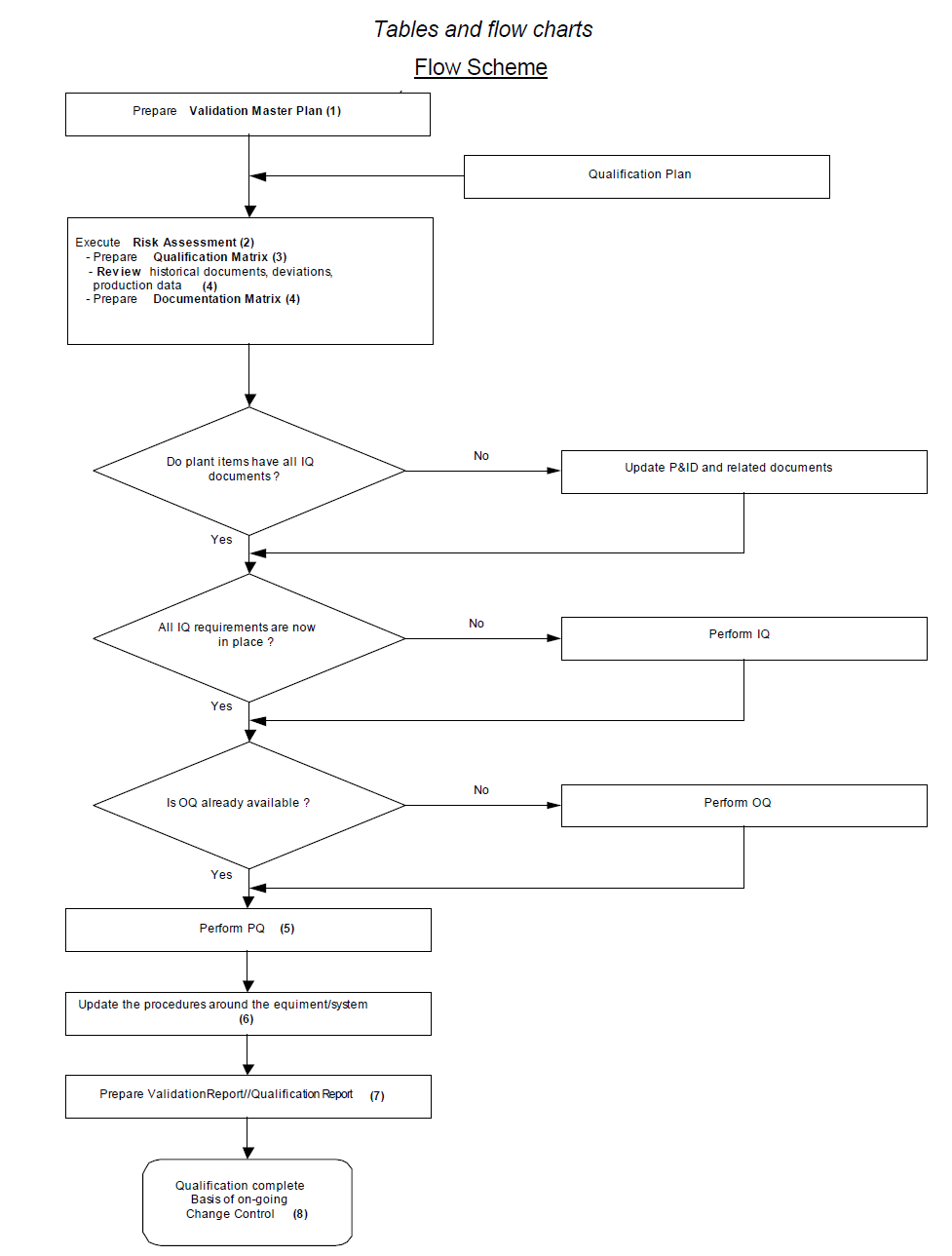
- Collect flow diagrams and matrixes which can be useful in providing an overview and monitoring tool. A flow scheme for the introduction of a new product into an existing plant is shown below.
2.0 Refer to the below table which indicates the activities in the qualification.
Table
Flow Scheme of Activities in the Qualification
| SNo. | Activity | Examples | Document |
| 1.0 | Define qualification scope including systems and equipment | – | VMP (QP) |
| 2.0 | Execute Risk Assessment (RA) | – | RA matrix |
| 3.0 | Set-up of a Qualification Matrix with all systems, components | – | Qualification Matrix (IQ) |
| 4.0 | Review historical documents and production data | – Datasheet(s) / Technical Description / Certificates (e.g. material of construction) – Drawing(s) – Parts list(s) – Installation instructions – Operating instructions – Maintenance instructions and records – Calibration instructions and records – Copy of supplier’s proposal, order and order confirmation – Commissioning and test plans – Electrical diagrams – Batch records and analytical results and plant parameter data |
Engineering Files and Documentation MatrixProcess Validation and Production Campaign Reports |
| Collect and review all historical deviations, changes related to equipment/systems, and failures if available | – Deviation Reports Change Reports Complaint Files | Evaluation Report Annual Product Review | |
| Reviewing the available procedures around the equipment/system | – Maintenance, calibration procedures | SOP’s and working instructions | |
| 5.0 | Review the qualification/accuracy of equipment and implement OQ/PQ | OQ, PQ | Qualification Matrix (OQ, PQ) |
| 6.0 | Updating the available procedures around the equipment/system | Maintenance, calibration, and operating procedures. | SOP’s and working instructions |
| 7.0 | Prepare Validation Report / Qualification Report | – | Validation Master Report (or equivalent reports) |
| 8.0 | Have basis for on-going Change Control | – | – |
3.0 Prepare a Validation Master Plan (VMP) and/or Qualification Plan (QP). A section of the Validation Master Plan should cover the qualification of plant and equipment.
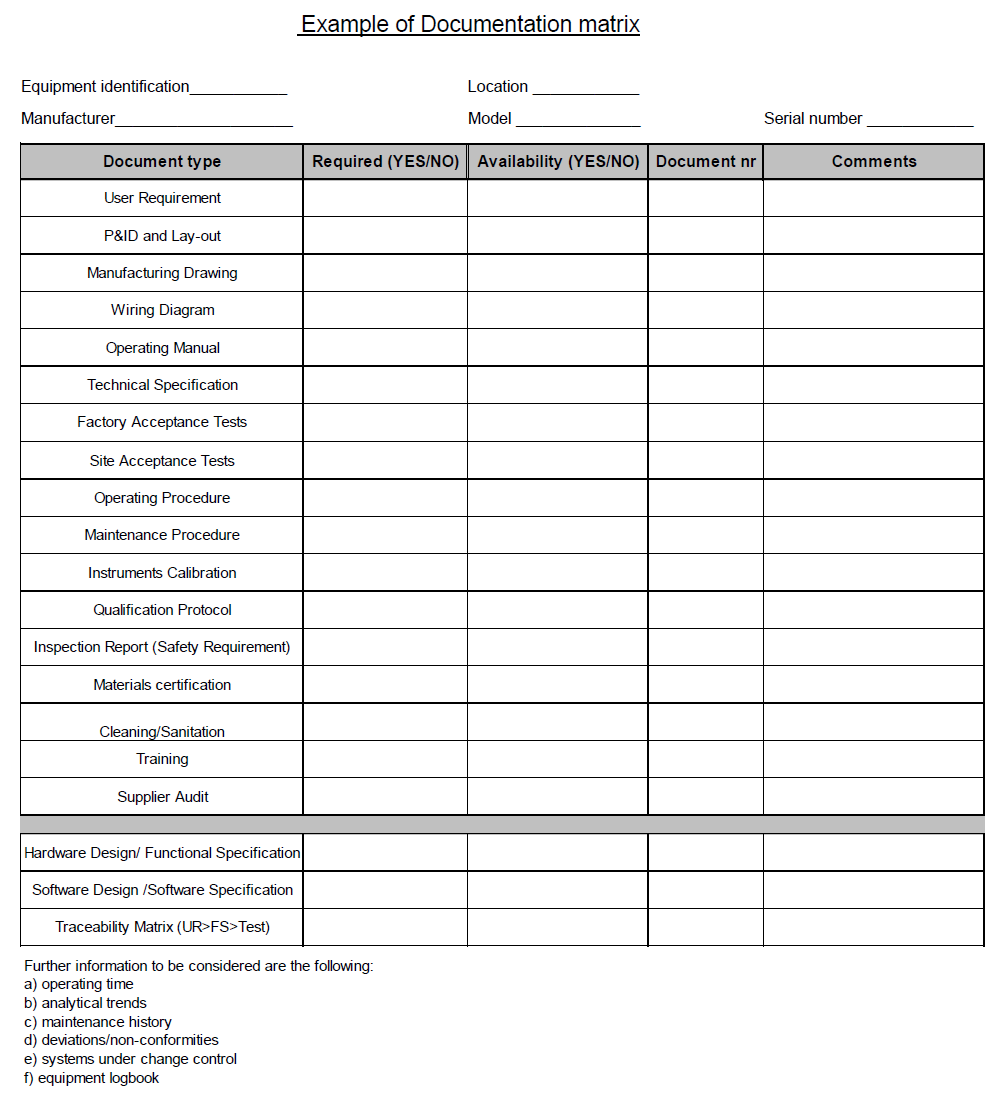
4.0 Collect, review and update related documentation to the equipment (procedures, change control, historical production data, process deviations). This activity may be done using a documentation matrix, example as shown below-
5.0 Execute the Risk Assessment Process to identify which items of equipment require qualification. The Risk Assessment Process will be carried out on every system or sub-system.
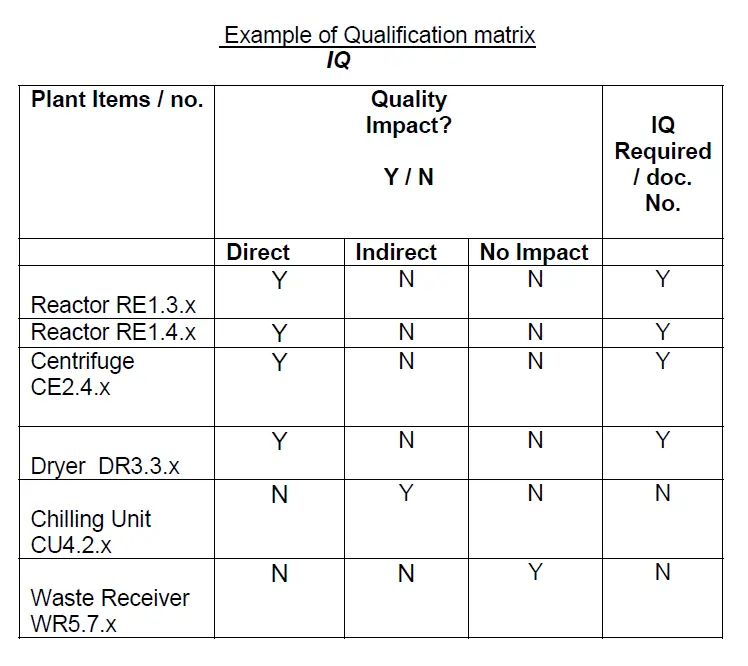
6.0 Prepare a Qualification Matrix for IQ. Please see below –
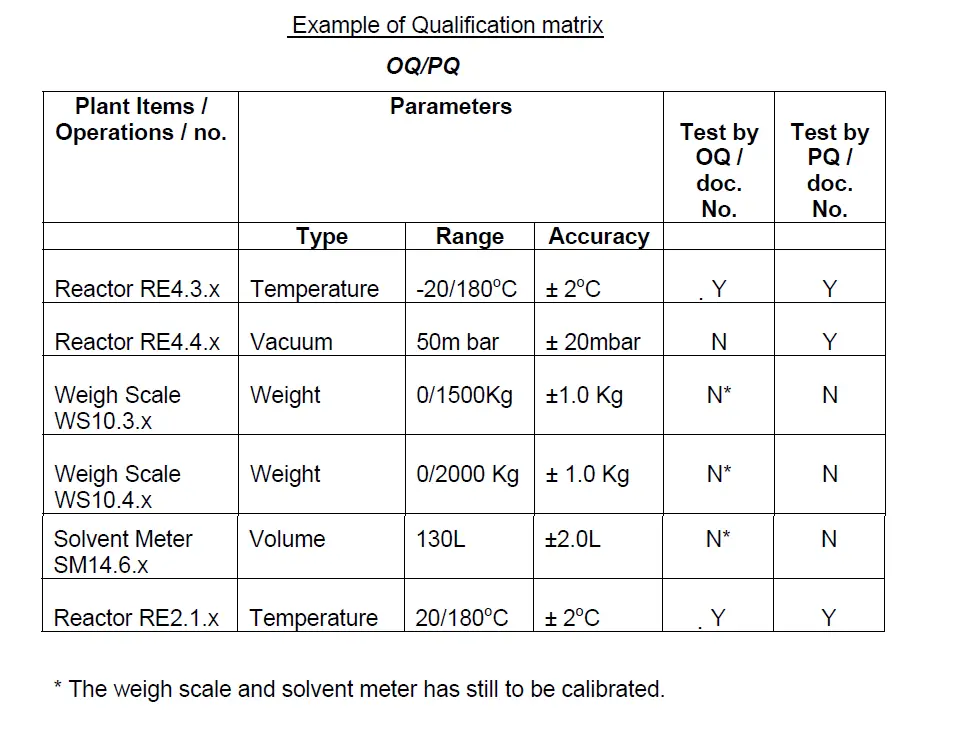
The weigh scale and solvent meter has still to be calibrated.
The weigh scale and solvent meter have still to be calibrated.
7.0 Update the detailed line diagram of the plant by checking it against the physical plant. The existing Installation Qualification (IQ) and engineering files should be reviewed and updated.
8.0 Prepare a further Qualification Matrix to review the accuracy and range of equipment/ instruments and identify items requiring OQ and PQ . Please see below –
9.0 Perform the OQ and PQ to complete the qualification.
10.0 Ongoing change management should be implemented after reporting and approval of all activities in the VMP and/or QP.
Reference: Guidance on Qualification of existing facilities, systems, equipment and utilities(APIC)