Preformulation for Tablets, Capsules, Liquid Orals
Before developing a formulation like tablets, capsules, liquid orals we study the suitability of new drug or drug and excipients for the chosen formulation which is called preformulation.
Preformulation Definition
Preformulation may be defined as a stage of the research and development process where the preformulation scientist characterizes the physical, chemical, biopharmaceutical and mechanical properties of a new drug substance, in order to develop stable, safe and effective dosage form.
Preformulation Factors
It is study about physical and chemical properties of drug substance prior formulation is called as preformulation.
They are:
• pH /pka
• Solubility
• Thermal/heat effect
• Dissociation constant
• Compatabilty studies – FTIR / DSC
• Oxidation and reduction
• Particle size
PHYSICAL PROPERTIES
It is vital to understand the physical description of a drug substance (whether it is solid, semisolid or liquid) prior to dosage form development. Most drugs in use nowadays are solid materials and less number are liquid in nature. Flowability of powder and chemical stability depends on the habit and internal structure of a drug.
Principal Areas of Preformulation :
(I) Nature of Solid Drug:
1. Crystallinity and polymorphism
2. Hygroscopicity
3. Fine particle characterization
4. Powder flow
(II) Solubility Data:
1. Ionization constant – pKa v (- log Ka)
2. pH solubility profile
3. Common ion effect – KSP.
4. Thermal effects
5. Solubilization
6. Partition coefficient
7. Dissolution
(III) Stability Analysis:
1. Stability in toxicology formulation
2. Solution stability
(a) pH stability profile
3. Solid state stability
(a) Bulk stability
(b) Compatibility
Nature of Solid Drug
When a drug molecule is invented, all the solid-forms are hardly identified. So, during bulk characterization the following characteristics are studied.
1. Crystallinity and Polymorphism:
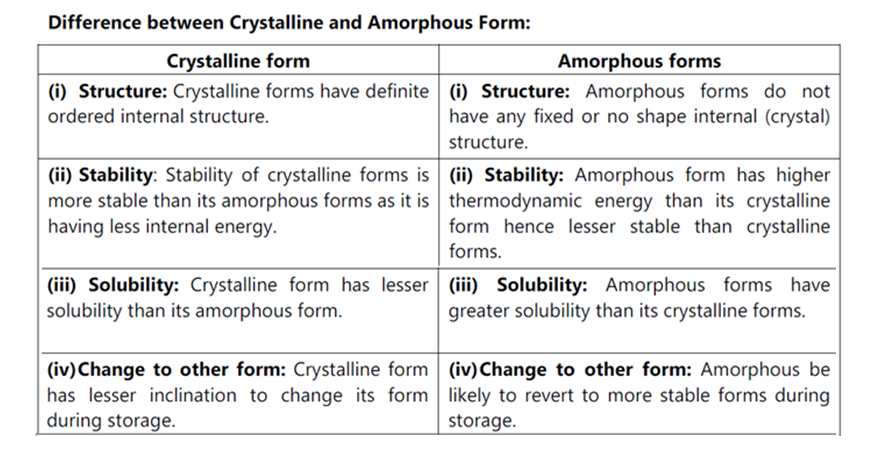
Crystal habit (external shape of crystal) is the description of the outer appearance of a crystal. A single internal-structure for a compound can have many different habits, depending on the environment for growing crystals. Different habits of crystals are given below. Internal Structure of drug may be crystalline and amorphous forms.
(i) Crystalline state:
In this state of matter atoms or molecules are arranged in highly well-ordered form and is associated with three-dimensional periodicity to organize themselves into their most favourable thermodynamic state, which under certain conditions results in their appearance as crystals. The repeating three-dimensional patterns are called crystal lattices.
The crystal lattice can be analyzed from its X-ray diffraction pattern.
Crystal Habit: Platy, Needle, or Acicular, Tabular, Equant or Massive, Bladed, Prismatic
Amorphous forms: In this form the solids do not have any definite internal structure.They have atoms or molecules disorderly placed as in a liquid. e.g. Amorphous Itraconazole, Amorphous Novobiocin
General Preparation: Amorphous forms are prepared by rapid precipitation, lyophillization or rapid cooling of molten liquids e.g. glass.
(ii) Polymorphs: When a substance is in more than one crystalline form, the various forms are called polymorphs and the phenomenon as polymorphism.
e.g. Chloramphenicol palmitate has three polymorphs: A, B and C. Spironolactone exhibits 6 polymorphs.
Various polymorphs can be prepared by crystallizing the drug from different drugs under various conditions. Depending on their relative stability, one of the different polymorphic forms will be physically more stable than the others. Such a stable polymorph represents the lowest thermodynamic energy state, has highest melting point and least solubility.
The representing polymorphs are called metastable forms which represent higher thermodynamic energy state; the metastable forms have a thermodynamic tendency to convert to the stable form. A metastable form cannot be called unstable because if it is kept dry, it will remain stable for years.
Molecular Adducts:
During the process of crystallization, some compounds have a tendency to trap the solvent molecules.
(a) Non-Stoichiometric Inclusion Compounds (or Adducts):
In these crystals solvent molecules are entrapped within the crystal lattice and the number of solvent molecules is not included in stoichiometric number.
Depending on the shape they are of three types:
(i) Channel: At a point when the crystal contains continuous channels in which the solvent molecule can be incorporated. e.g. Urea and Thiourea forms channel.
(ii) Layers: Here solvent molecules are ensnared in between layers of crystals. Some compounds, such as clay Montmorillonite, the principle constituents of bentonite, can entrap hydrocarbons, alcohols and glycols between the layers of their lattices.
(iii) Clathrates (Cage): Solvent molecules are entrapped within the cavity of the crystal from all sides.eg Hydroquinone
(b) Stoichiometric Inclusion Compounds (or Stoichiometric Adducts):
This molecular complex has consolidated the crystallizing solvent molecules into specific sites within the crystal lattice and has stoichiometric number of solvent molecules complexed.
At the point when the merged solvent is water, the complex is called hydrates and when the solvent is other than water, the complex is called solvates.
Depending on the ratio of water molecules within a complex the following nomenclature is followed.
(i) Anhydrous: 1 mole compound + 0 mole water e.g. Ampicillin
(ii) Hemihydrate: 1 mole compound + ½ mole water
(iii) Monohydrate: 1 mole compound + 1 mole water
(iv) Dihydrate: 1 mole compound + 2 moles water
(v) Trihydrate: 1 mole compound + 3 moles water e.g. Ampicillin Trihydrate
Properties of Solvates/Hydrates:
(i) Generally, the anhydrous form of a drug has more prominent fluid solvency than its hydrates. This is on the basis that the hydrates are already in equilibrium with water and therefore have less demand for water. e.g. anhydrous forms of theophylline and ampicillin have higher aqueous solubility than the hydrates.
(ii) Non aqueous solvates have greater aqueous solubility than the non-solvates. E.g. chloroform solvates of griseofulvin are more water soluble than their nonsolvate forms.
Analytical Methods for Characterization of Solid Forms:
Methods of studying solid forms are listed as below (amount of drug required for study):
(a) Microscopy (1 mg)
(b) Hot stage microscopy (1 mg)
(c) Differential Scanning Calorimetry (DSC) (2 – 5 mg)
(d) Differential Thermal Analysis (DTA) (2 – 5 mg)
(e) Thermogravimetric Analysis (10 mg)
(f) Infrared Spectroscopy (2 – 20 mg)
(g) X-ray Powder Diffraction (500 mg)
(h) Scanning Electron Microscopy (2 mg)
(i) Dissolution / Solubility Analysis (mg – g)